Synthesis and application of novel cyclodextrin polymer chiral resolving agents
A technology of cyclodextrin polymer and cyclodextrin, which is applied in the direction of electrogenesis process, electrolysis process, etc., can solve the problem of high ion strength of charged CDs derivatives, high ion strength of charged cyclodextrin derivatives, degree of substitution and substitution position Difficult to control and other problems, to achieve good economic value and application prospects, convenient polymer purification treatment, strong chiral resolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
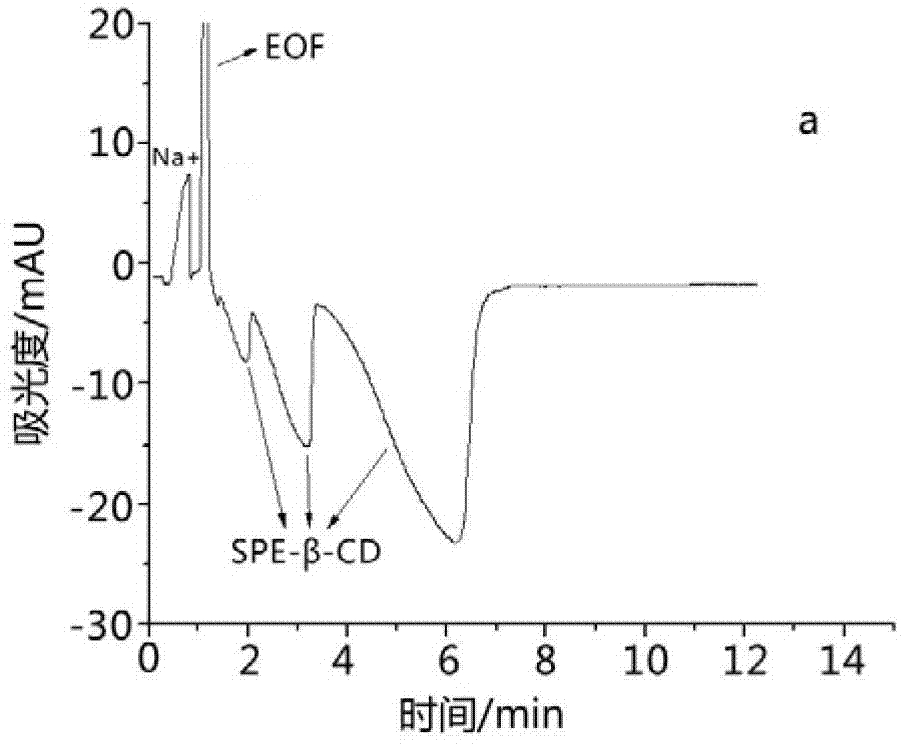
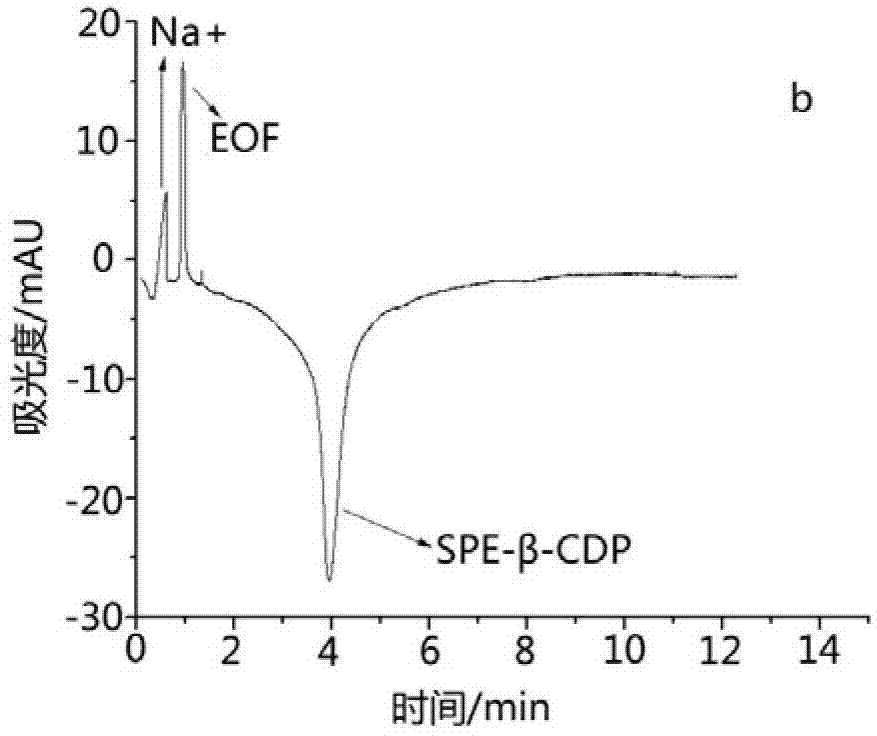
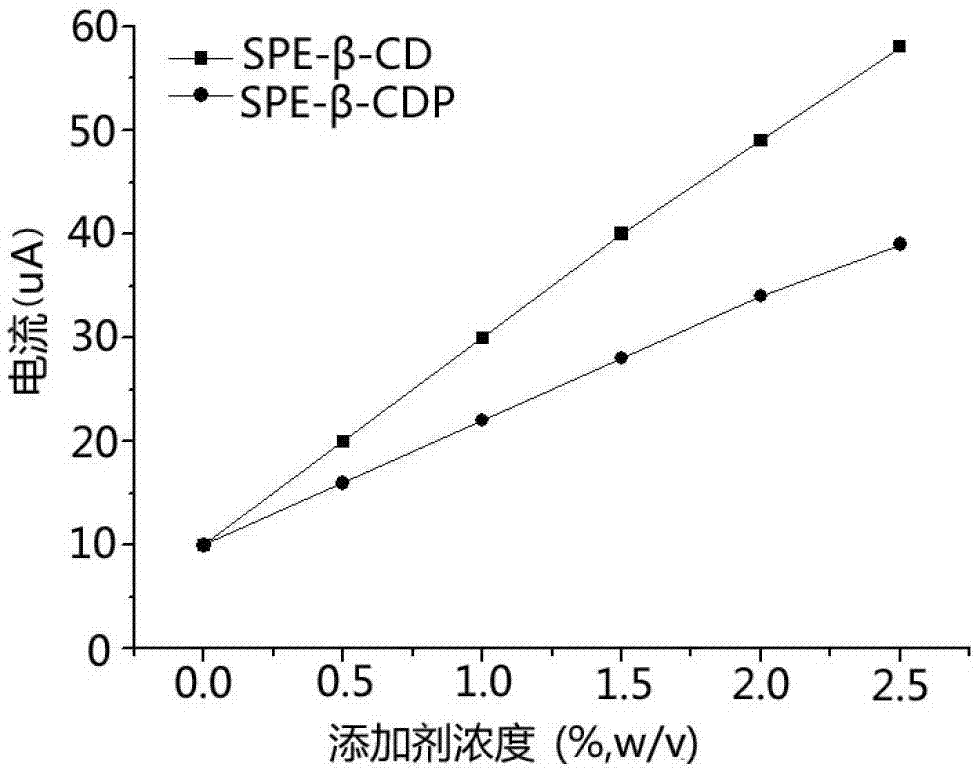
Image
Examples
Embodiment 1
[0056] Example 1: Synthesis of SPE-β-CDP according to the reaction steps of first sulfonation and then polymerization
[0057] 9 g of recrystallized β-CD was slowly added to 20 mL of 20% (w / v) NaOH solution. After stirring at room temperature for 1 h, 4.2 mL of 1,3-propane sultone (1,3-PS) was slowly added dropwise, and the mixture was reacted at 75° C. for 3 h. After stopping the reaction, cool to room temperature, neutralize to neutral with 6 mol / L HCl, concentrate the reaction solution and dry to obtain 11.58 g of light yellow solid SPE-β-CD with a yield of 87.67%.
[0058] 4g of the above-mentioned sulfopropyl ether β-cyclodextrin was dissolved in 4mL of 30% (w / v) NaOH solution, and stirred for 0.5h until completely dissolved. Add 2 mL of epichlorohydrin (Ep), keep stirring rapidly, stop the reaction after reacting at 75 °C for 3 h, cool to room temperature, neutralize with 6 mol / L HCl, pour into a dialysis bag with a molecular cut-off of 7000, and remove Dialyze in deio...
Embodiment 2
[0059] Example 2: Synthesis of SPE-α-CDP according to the reaction steps of first sulfonation and then polymerization
[0060] Weigh 3.0000g α-cyclodextrin into a round bottom flask, add 6.0mL 20% (w / v) NaOH solution, stir and dissolve at room temperature for 0.5h, add 2.6mL 1,3-PS, react at 75°C for 3h After the reaction is complete, cool to room temperature and adjust the pH to neutral with dilute HCl. The concentrated reaction solution was dried to obtain 6.2162 g of light yellow solid SPE-α-CD.
[0061] Weigh 3.0064g of sulfopropyl ether α-cyclodextrin monomer into a round bottom flask, add 6.0mL of 30% (w / v) NaOH solution, stir and dissolve at room temperature for 0.5h, add 4mL of Ep, at 75°C After reacting for 3 h, the reaction was cooled to room temperature, and the pH was adjusted to neutral with dilute HCl. Dialysis was performed with a dialysis membrane with a molecular cut-off of 7000 to remove unreacted monomers, and the dialysate was concentrated and dried by ro...
Embodiment 3
[0062] Example 3: Synthesis of SPE-γ-CDP with different degrees of polymerization according to the reaction steps of first sulfonation and then polymerization
[0063] Weigh 10g of γ-CD and slowly add it into 20mL of 20% (w / v) NaOH solution. After stirring at room temperature for 0.5 h, 7.7 mL of 1,3-PS was slowly added dropwise, and the mixture was reacted at 75° C. for 3 h. Cool down after stopping the reaction, neutralize with 6 mol / L HCl, concentrate the reaction solution and dry to obtain 20.9536 g of light yellow solid SPE-γ-CD.
[0064] Then synthesize the following SPE-γ-CDP with different degrees of polymerization:
[0065] (1) 2.0057g of the above SPE-γ-CD was dissolved in 1.8mL of 30% (w / v) NaOH solution, stirred for 0.5h until completely dissolved. Add 0.6mL (5eq) Ep, keep stirring rapidly, stop the reaction at 75°C for 3h, cool to room temperature, neutralize with 6mol / L HCl, pour into a dialysis bag with a molecular weight cut off of 7000, and dialyze in deioni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com