Method for determining contents of carbohydrate components in compound Salvia miltiorrhiza extract
A compound salvia miltiorrhiza and a method for determination are applied in the field of content determination of carbohydrates in compound salvia miltiorrhiza extracts, and can solve the problems of low sensitivity, poor stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Determination method of carbohydrate components in compound salvia miltiorrhiza extract:
[0069] Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition).
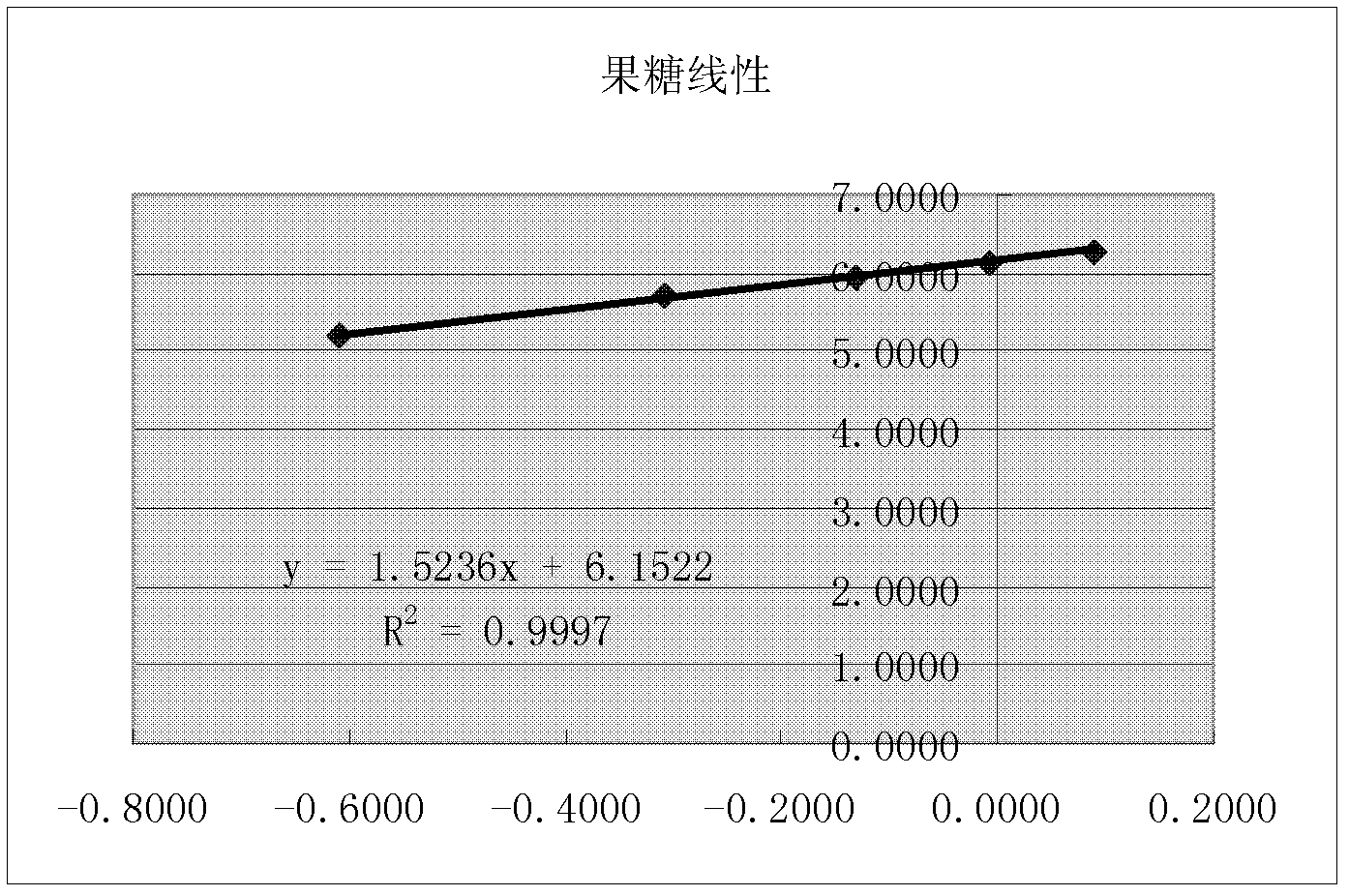
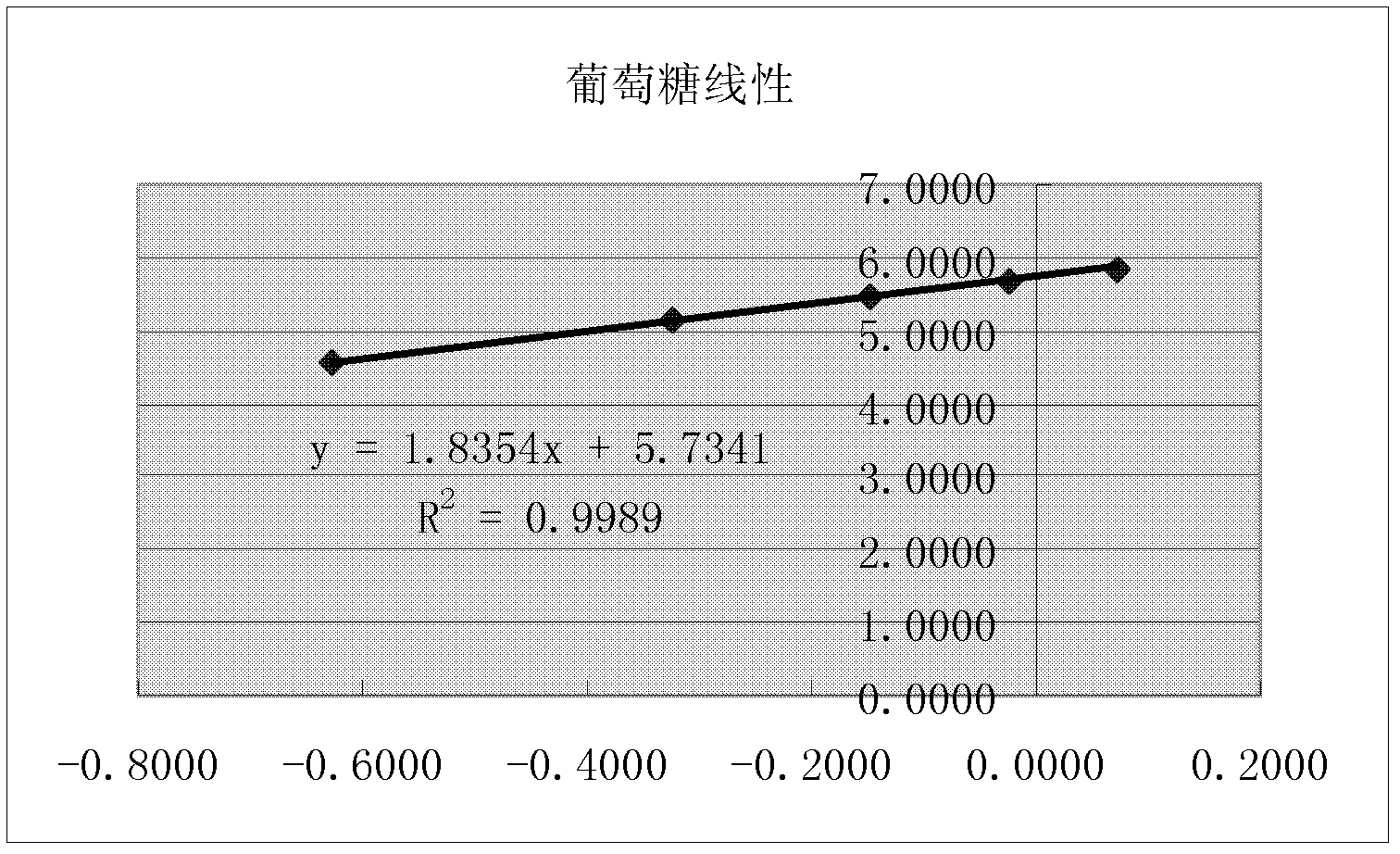
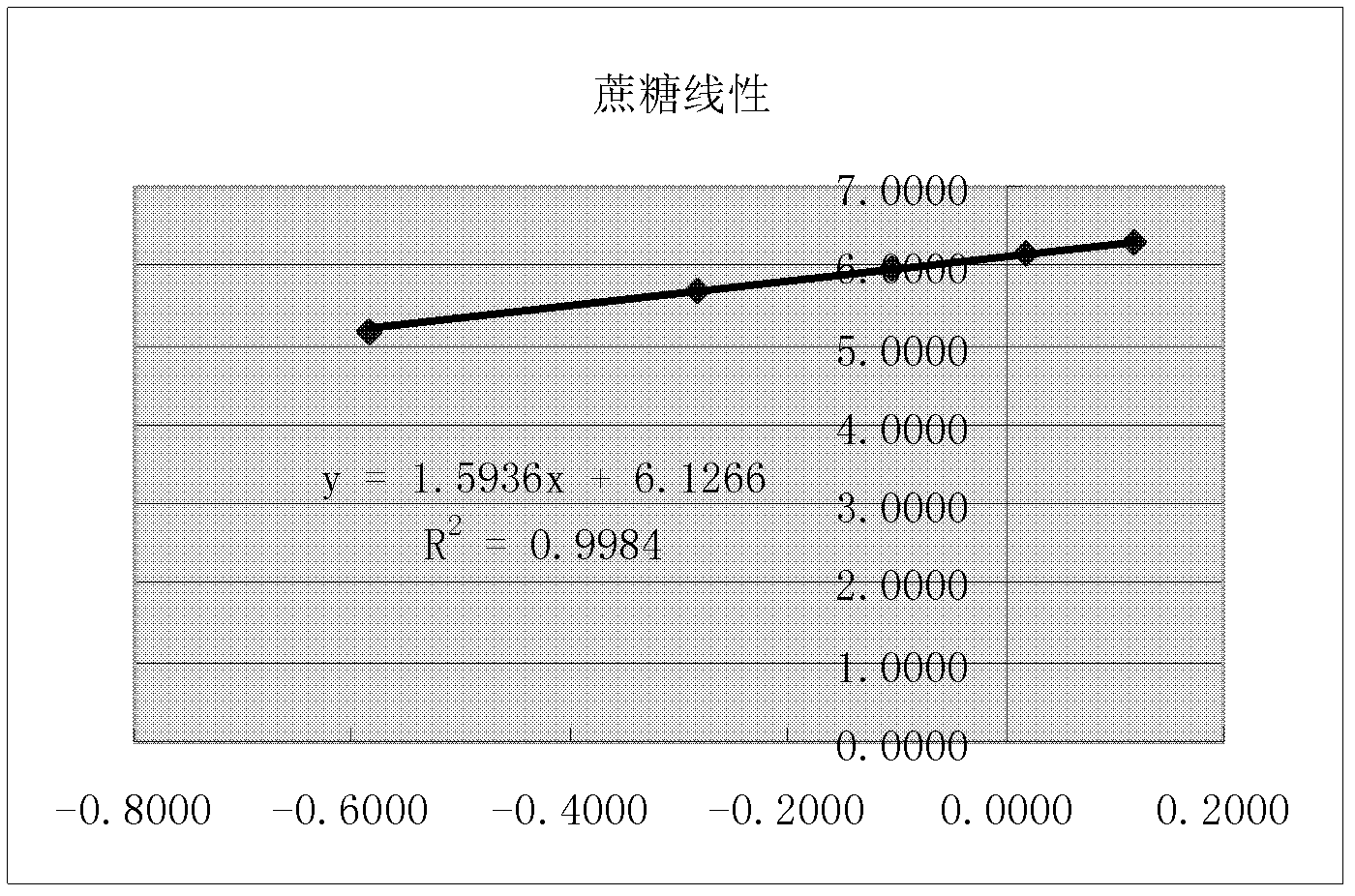
[0070] The chromatographic conditions and system suitability test used PrevailTM Carbohydrate ES chromatographic column, with acetonitrile as mobile phase A, water as mobile phase B, flow rate 0.8ml / min, column temperature 30°C; WATERS 2420ELSD detector was used for detection, and the parameter was set to gain 10. Air pressure 25psi, drift tube 60°C, Neb heater: 60%. The number of theoretical plates should be no less than 4000 based on the chromatographic peak of sucrose, and the chromatographic resolution of fructose and glucose should be no less than 2.0.
[0071] time (minutes)
water
0
75
25
20
75
25
23
60
40
30
60
40
33
75 ...
Embodiment 2
[0076] Recipe: Salvia 20%, Panax notoginseng 79%, Borneol 1%.
[0077] Step 1: decoct the salvia miltiorrhiza and notoginseng with 5 times the weight of alkaline water with a pH value of 8.3 for 2 hours, filter, add 4 times the amount of water to the dregs of the medicine and decoct for 1 hour, and mix the extract.
[0078] Step 2, the extract is concentrated and dried.
[0079] Determination method of carbohydrate components in compound salvia miltiorrhiza extract:
[0080] Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition).
[0081] Chromatographic conditions and system suitability test using PrevailTM Carbohydrate ES chromatographic column, with acetonitrile as mobile phase A, water as mobile phase B, flow rate 0.8ml / min, column temperature 30°C; detection with Alltech ELSD2000 detector, gas flow rate: 2.5 ml / min, drift tube 105°C. The number of theoretical plates calculated based on the sucrose chromatogra...
Embodiment 3
[0086] Formula: Salvia 97%, Panax notoginseng 2%, Borneol 1.0%.
[0087] Step 1: decoct the salvia miltiorrhiza and notoginseng with 5 times the weight of alkaline water with a pH value of 8.3 for 2 hours, filter, add 4 times the amount of water to the dregs of the medicine and decoct for 1 hour, and mix the extract.
[0088] Step 2: Concentrate the extract, precipitate with alcohol, separate the supernatant, and filter.
[0089] Step 3, recovering ethanol and drying.
[0090] Determination method of carbohydrate components in compound salvia miltiorrhiza extract:
[0091] Determine according to high performance liquid chromatography (Appendix VI D of Chinese Pharmacopoeia 2010 edition).
[0092] The chromatographic conditions and system suitability test used PrevailTM Carbohydrate ES chromatographic column, with ethanol as mobile phase A, water as mobile phase B, flow rate 0.8ml / min, column temperature 30°C; WATERS 2420ELSD detector was used for detection, and the parameter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com