Method for preparing fused salt-oxalate co-precipitation of lithium-rich materials for lithium batteries
A lithium-rich material, molten salt technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of precipitation speed and particle size uniformity that are not easy to ensure, and achieve the stability of material structure, improve electrochemical performance, The effect of uniform particle size of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
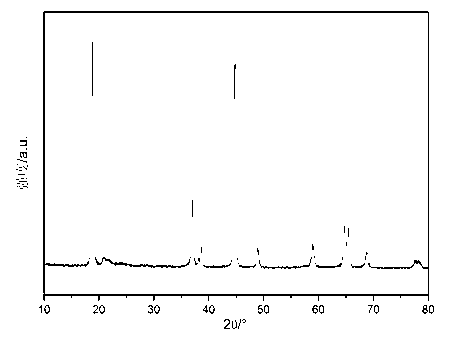
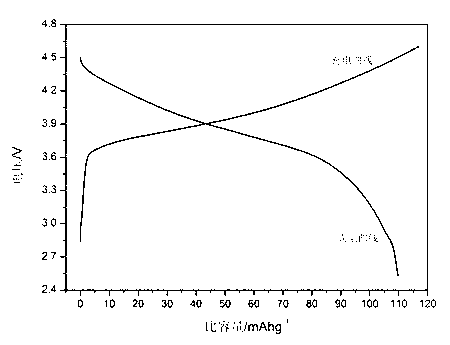
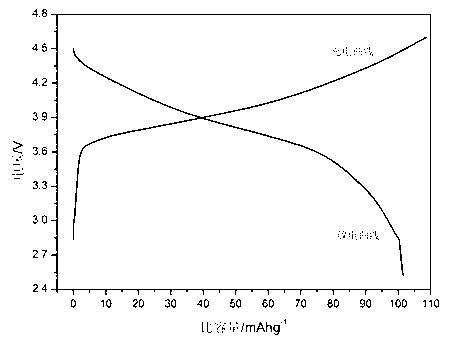
Image
Examples
Embodiment 1
[0026] Preparation of Li[Li 0.33 Ni 0.33 mn 0.56 ]O 2 material, namely xLi 2 MnO 3 ( 1-x )LiMO 2 M=Ni in the material, x = 0.33. First, 9.693 g of Ni(NO 3 ) 2 and 9.941 g of Mn(NO 3 ) 2 Dissolve in 220 mL of deionized water to obtain solution A, and dissolve 6.75 g of oxalic acid in 150 mL of deionized water to obtain solution B. Take another beaker C, add 150 mL of deionized water, use ammonia water to control the pH at 8, and use a peristaltic pump to simultaneously drop liquids A and B into beaker C at a rate of 0.2 mL / min in a water bath at 40 °C. and stir vigorously. The obtained suspension was filtered, washed, and air-dried to obtain powder D. LiOH and LiNO with a molar mass ratio of 3.8:6.2 3 After grinding and mixing, heat it to 200°C to melt it, keep it warm for 1 hour, take out the crystal and grind it to get molten salt LiOH-LiNO 3 , coded as molten salt E. Mix 9.2921 g of molten salt E, 3.19 g of LiOH and D by ball milling to obtain mixture F. P...
Embodiment 2
[0028] Preparation of Li[Li 0.184 Ni 0.224 mn 0.725 ]O 2 material, namely x Li 2 MnO 3 ( 1-x )LiMO 2 M= Ni in the material 1 / 2 mn 1 / 2 , x = 0.45. First, 5.888 g of NiSO 4 and 12.253 g of MnSO 4 Dissolved in 120 mL deionized water to obtain solution A, 15g Na 2 C 2 o 4 Dissolve in 200 mL deionized water to obtain solution B. Use hydrochloric acid to control the pH value of solution B to be stable at 3, add solution A dropwise to solution B at a rate of 3 mL / min in a water bath at 80°C, and stir vigorously, filter the obtained suspension, wash and vacuum Powder C was obtained after drying. 34.79 g KCl, 8.152 g LiNO 3 Mix with ball mill C to get mixed powder D, put D into a resistance furnace, heat up to 600°C at a rate of 50°C per minute and keep it warm for 9 hours, then cool with the furnace, the resultant is washed repeatedly with deionized water and then baked at 60°C Dry to obtain the final product.
Embodiment 3
[0030] Preparation of Li[Li 0.167 Ni 0.166 co 0.166 mn 0.500 ]O 2 material, namely x Li 2 MnO 3 ( 1-x )LiMO 2 M= Ni in the material 1 / 3 co 1 / 3 mn 1 / 3 , x = 0.4. First, 4.13 g of Ni(CH 3 COO) 2 , 0.243 g of MnCl 2 and 4.135 g of Co(CH 3 COO) 2 Dissolved in 200 mL deionized water to obtain solution A, 26.8g K 2 C 2 o 4 Dissolve in 100 mL deionized water to obtain solution B. Under hydrothermal conditions at 65°C, use a peristaltic pump to drop B into solution A at a rate of 10 mL / min, while adjusting its pH to 6 with hydrochloric acid and stirring vigorously. The resulting suspension was filtered, washed and dried in vacuo to afford C. 44.06 g Li 2 CO 3 Mix and grind with powder C to obtain mixture D. Put D into a resistance furnace, raise the temperature to 900°C at a rate of 10°C per minute, keep it warm for 4 hours, and then cool it with liquid nitrogen. The resultant was washed several times with deionized water and then dried at 70°C to obtain t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com