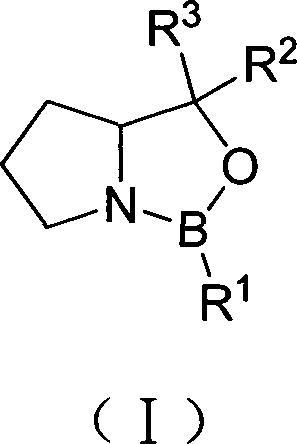
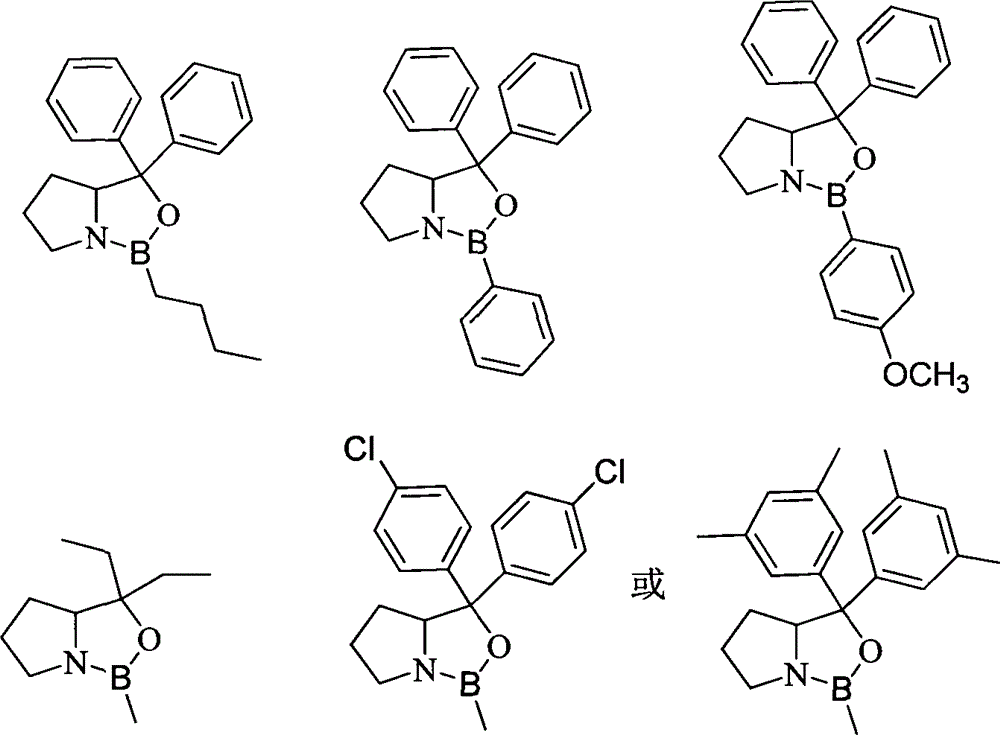
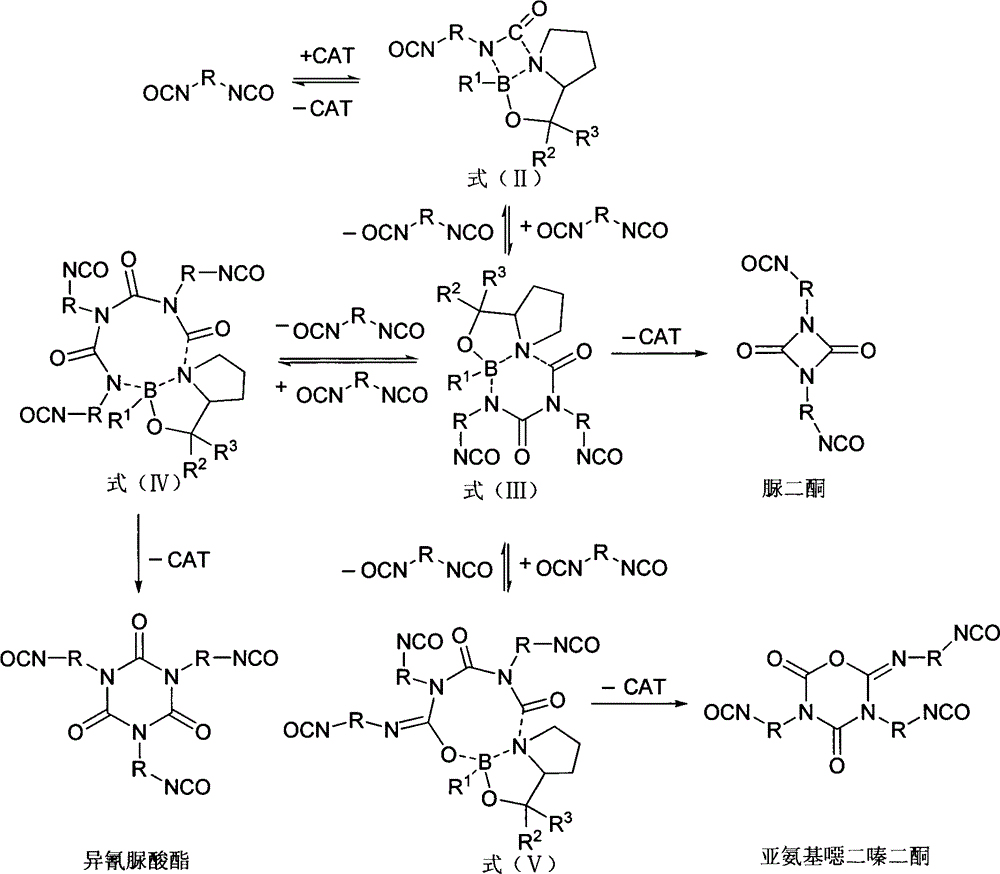
Method for preparing polyisocyanate containing uretidione group
A technology of polyisocyanate and uretdione group, which is applied in the field of preparing polyisocyanate with high content of uretdione group, which can solve the problems of air instability, product coloring, and product coloring of tertiary phosphine compounds, and improve raw materials Conversion-dependent, low-color effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1kg (5.95mol) of hexamethylene-1,6-diisocyanate (HDI) was placed in a four-necked flask, at 60°C, 2.98ml of catalyst a in toluene solution (2mol / L) was added under stirring conditions, and the timing was started . During the reaction process, the reaction temperature was controlled at 60° C. to 70° C., HDI was quantified by gel chromatography, and the conversion rate of the reaction was monitored. When the specified conversion rate was reached, 11.92 mmol of diethyl phosphate was added to terminate the reaction. After the reaction, it is separated to obtain HDI polyisocyanate with light color and high uretdione content.
Embodiment 2-3
[0042] Except using the toluene solution (4mol / L) of 7.45ml catalyst b, c to catalyze the oligomerization reaction successively, 35.76mmol diethyl phosphate terminates the reaction, others are the same as embodiment 1.
Embodiment 4-6
[0044] Except using 59.5mmol of catalysts d, e, f to catalyze the oligomerization reaction successively, and 59.5mmol of diethyl phosphate to terminate the reaction, the others were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com