Engineered opsonins for pathogen detection and treatment
An opsonin, collagen technology, applied in the systemic field of pathogens, can solve problems affecting the complement system and agglutination pathways, vasculature and organ damage, death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Construction and expression of embodiment 1.FcMBL.81
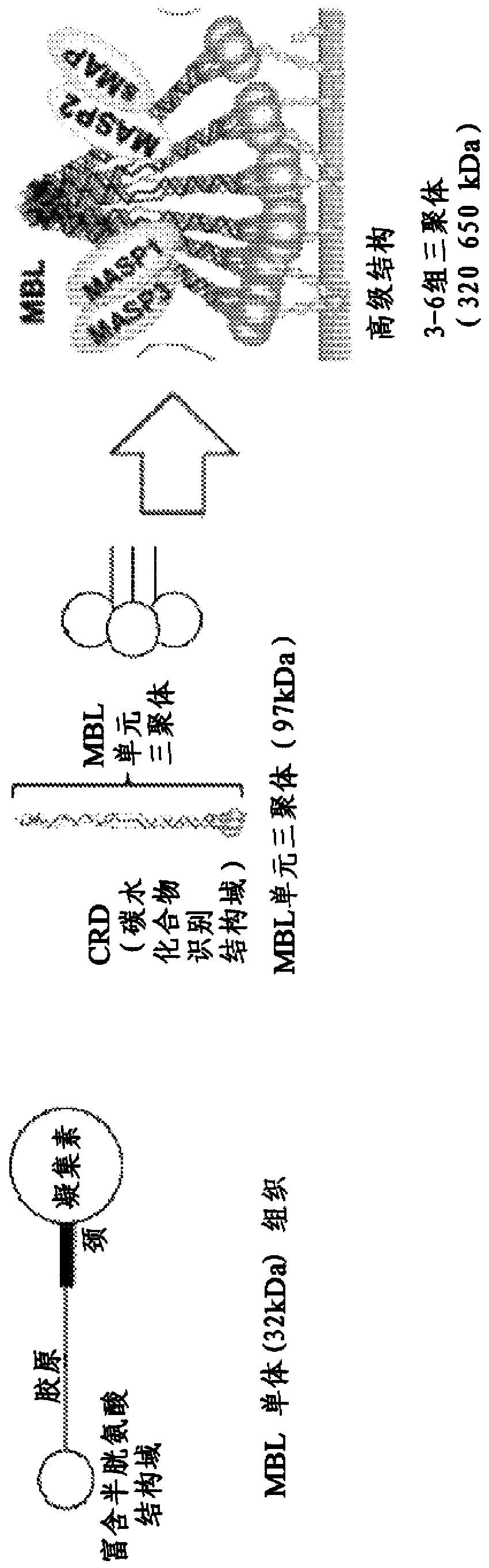
[0108] An embodiment of an engineered construct MBL for use as a general opsonin in diagnostic and therapeutic applications can be constructed using the "neck" and "lectin" domains of MBL. The proline at position 81 (as defined in Structural Bioinformatics Research Center, Protein Data Bank Structure File 1HUP) was chosen as the N-terminal point at which the lectin sequence begins. This localized downstream (C-terminus) of the lectin molecule is fused to the human Fc portion γ1 (Fcγ). exist image 3 A diagram of the engineered lectin construct is shown in . exist Figure 4 A schematic diagram of the cloned Fc portion is shown in .
[0109] The amino acids of this construct include the following residues:
[0110] Fc protein sequence:
[0111]
[0112] MBL.81 protein sequence (this sequence includes the coiled-coil neck region and carbohydrate recognition domain (CRD) of human MBL):
[0113]
[0114] Fc-...
Embodiment 2
[0118] Example 2. Comparison of Fc MBL.81 constructs with full length MBL for binding to yeast.
[0119] Approximately 5.5 million Candida albicans yeast cells were inoculated with varying numbers of MBL beads coated with wild-type full-length MBL (hexamer of trimers) or Fc MBL.81. like Figure 9 As shown in the figure, 18 million wild-type full-length MBL or Fc MBL.81 beads bound all 5.5 million fungal cells. This example demonstrates that Fc MBL.81 beads are as active as wild-type full-length MBL beads in binding to C. albicans.
[0120]
[0121]
[0122]
[0123]
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com