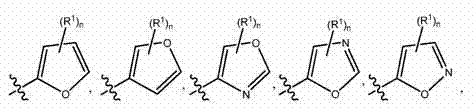
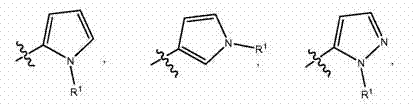
Indazole inhibitors of kinase
A technology of aryl and heterocyclic groups, which is used in medical preparations containing active ingredients, drug combinations, sexual diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
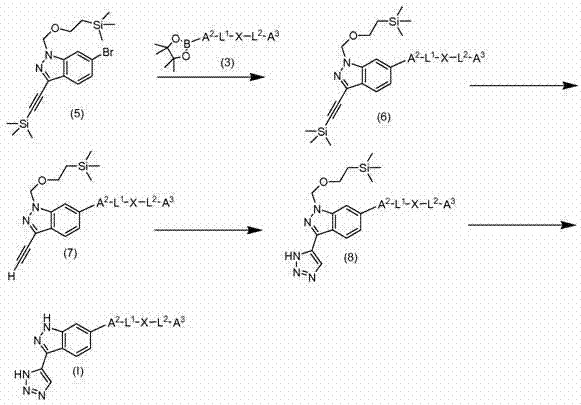
Embodiment 1
[0320] A solution of 6-bromo-1H-indazole (10 g, 50.8 mmol, commercially available) in dioxane (200 ml) was treated with 3N aqueous NaOH (100 ml). The well stirred mixture was treated with iodine (27.1 g, 107 mmol), added in portions over 5 min, then stirred for 60 min. with 200 ml of 20% citric acid solution, followed by 160 ml of saturated NaHSO 3 The solution was quenched before partitioning between ethyl acetate and water. with MgSO 4 The organic extracts were dried and concentrated to a solid which was triturated with ether and pentane to afford the title compound.
Embodiment 1A
[0322] 6-Bromo-3-iodo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazole
[0323] Example 1A (14.4 g, 44.6 mmol) was added to a 0° C. aqueous solution of potassium hydroxide (50.0 g, 892 mmol) in 200 mL of water. The concentrated suspension was stirred for 10 min and washed with CH 2 Cl 2 (400 ml) and treated with tetrabutylammonium bromide (1.437 g, 4.46 mmol). Then, (2-(chloromethoxy)ethyl)trimethylsilane (9.05 ml, 51.3 mmol) was added dropwise with a dropping funnel within 50 min. The reaction was stirred at 0 °C for 1.5 h and washed with CH 2 Cl 2 (2X) extracted and washed with water. with MgSO 4 The combined organics were dried, concentrated, and flashed by silica gel chromatography with CH 2 Cl 2 / hexane to obtain the title compound.
Embodiment 1B
[0325] 6-Bromo-3-(1-methyl-1H-pyrazol-4-yl)-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazole
[0326] Example 1B (0.500 g, 1.103 mmol) and 1-methyl-4-(4,4,5,5 - A solution of tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (0.275 g, 1.324 mmol) in toluene (8 ml) and ethanol (8 ml) . Add Pd(PPh 3 ) 4 (0.217 g, 0.188 mmol), and the resulting heterogeneous mixture was refluxed at 80°C for 2 hours and then stirred at room temperature for 18 hours. The reaction mixture was diluted with brine and extracted twice with ethyl acetate. with MgSO 4 The combined organics were dried, concentrated, and chromatographed on silica gel using 0.5% methanol in CH 2 Cl 2 The residue was purified by elution to obtain the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com