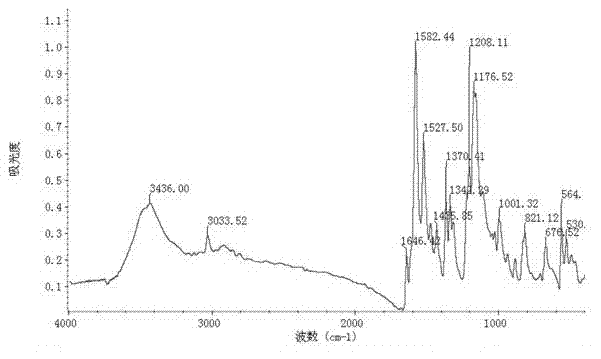
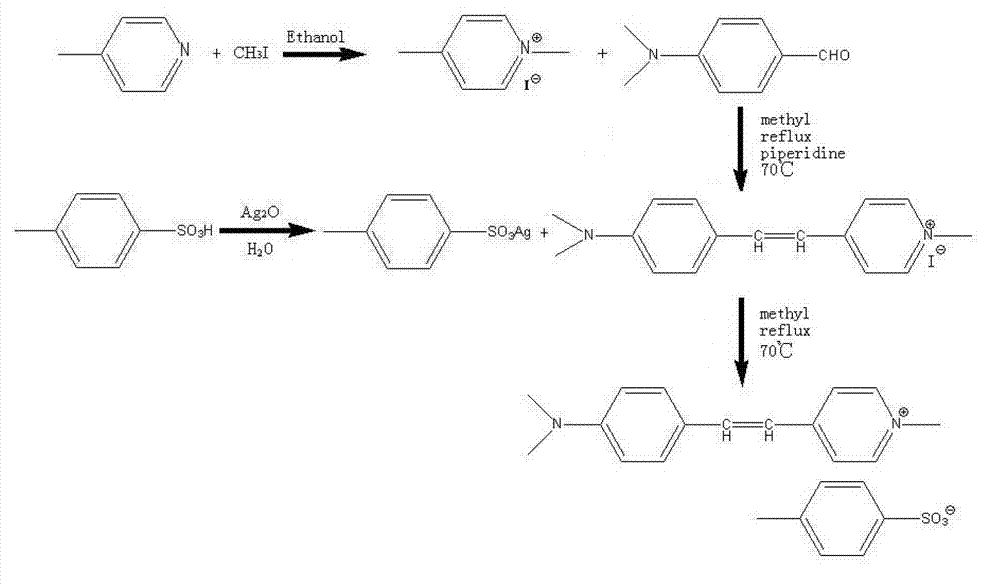
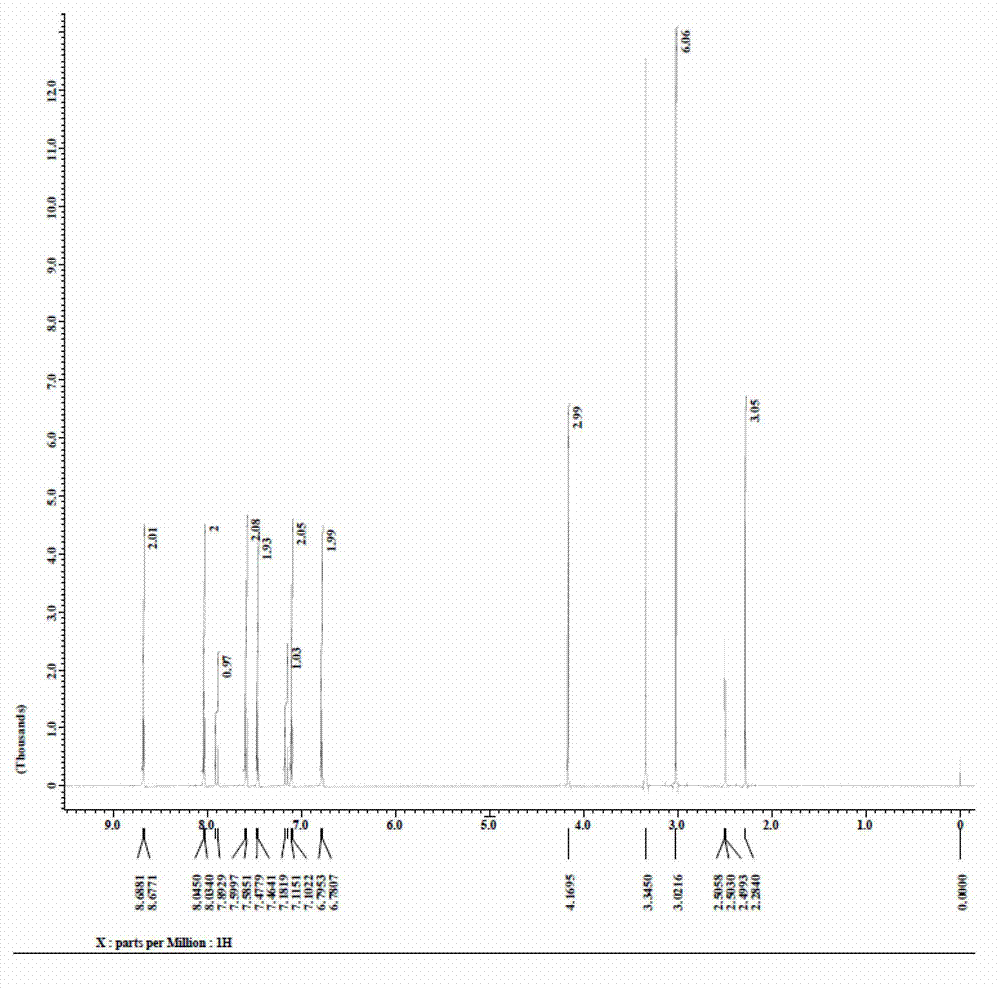
Preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate
A technology of dimethylaminostyrene and p-toluene sulfonate, which is applied in sulfonate preparation, organic chemistry and other directions, can solve the problems of limiting the preparation of high-quality nonlinear crystal materials, complicated purification process and high preparation cost, and achieves a reduction in The effect of experimental cost, high product purity and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0018] The specific steps for preparing 4-(4-dimethylaminostyryl)picoline p-toluenesulfonate in this example include five steps: synthesis reaction, condensation reaction, preparation of silver p-toluenesulfonate, exchange reaction and filtration purification:
[0019] (1) Synthesis reaction: Dissolve 0.03mol 4-picoline and 0.032mol iodomethane in 4ml and 4ml of absolute ethanol with a purity of 99.7%, respectively, and then mix and stir the two for 20 minutes to form a white needle-like solid. Utilize 99.7% ethanol to wash and filter, obtain 0.023mol 4-picoline iodide salt, yield rate is 76.7%;
[0020] (2) Condensation reaction: Dissolve 0.02mol of 4-picoline iodide and 0.02mol of p-dimethylaminobenzaldehyde in 20ml and 50ml of methanol with a purity of 99.5%, respectively, and then place the two solutions in 250ml of three In a well-boiled flask, heat and stir to 70°C, condense and reflux at the same time, add three drops of piperidine as a catalyst, the solution gradually ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com