Organic metal ruthenium compound and preparation method and application thereof
A ruthenium compound and organometallic technology is applied in the field of organometallic ruthenium compounds and their preparation, and achieves the effects of low cost, readily available raw materials and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
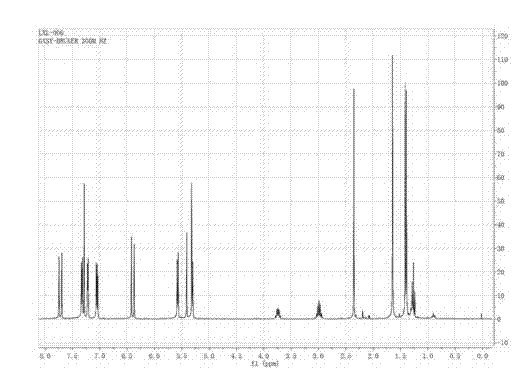
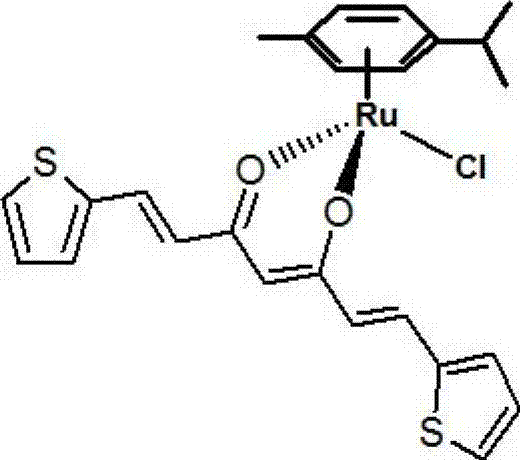
[0025] Preparation of organometallic ruthenium compounds
[0026] The preparation of organometallic ruthenium compound, its concrete steps are as follows:
[0027] 1) 0.366g ruthenium weight content is 37% RuCl 3 ·xH 2 O and 3ml of γ-terpinene with a purity of 95% were dissolved in 10ml of absolute ethanol, heated and stirred under reflux for 6 hours. The product A was obtained by standing and precipitating. 2) Weigh 0.091g of acetylacetone and 0.12ml of thiophene formaldehyde and dissolve them in 10ml of ethanol solution with a concentration of 50% by volume, heat to 80°C, and after 4 hours, let it stand for precipitation to obtain 1,7-di-2-thiophene -1,6-Heptadiene-3,5-dione.
[0028] 3) Dissolve 20mg, 0.2mmol of 1,7-di-2-thiophene-1,6-heptadiene-3,5-dione (20mg, 0,2mmol) and 32mg, 0.05mmol of product A in 8ml without Water and ethanol, heated and stirred to reflux for 6 hours, after the reaction was completed, the solution was evaporated to leave 2ml of liquid, and 30m...
Embodiment 2
[0030] In vitro anti-tumor activity test:
[0031] In vitro cytotoxicity assays were performed using the MTT method. The organometallic ruthenium compound obtained in Example 1 was reacted with gastric cancer SGC 7901 cell line for 72 hours respectively, and the results are shown in Table 2.
[0032] Table 2 The half maximal effective concentration (IC) of organometallic ruthenium compounds to tumor cell lines 50 )
[0033] cell line
[0034] From the results of Example 2, it can be seen that the organometallic ruthenium compound of the present invention has a strong anti-tumor activity through in vitro anti-tumor experiments. The invention provides a new idea for researching and developing new antitumor drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com