Liver disease marker, method and apparatus for measuring same, and test method for pharmaceutical preparation
A technology for markers and pharmaceuticals, which is applied in the field of liver disease markers, its determination, equipment and pharmaceutical inspection, and can solve problems such as glutathione consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0084] Hereinafter, embodiments of the present invention will be described in detail.
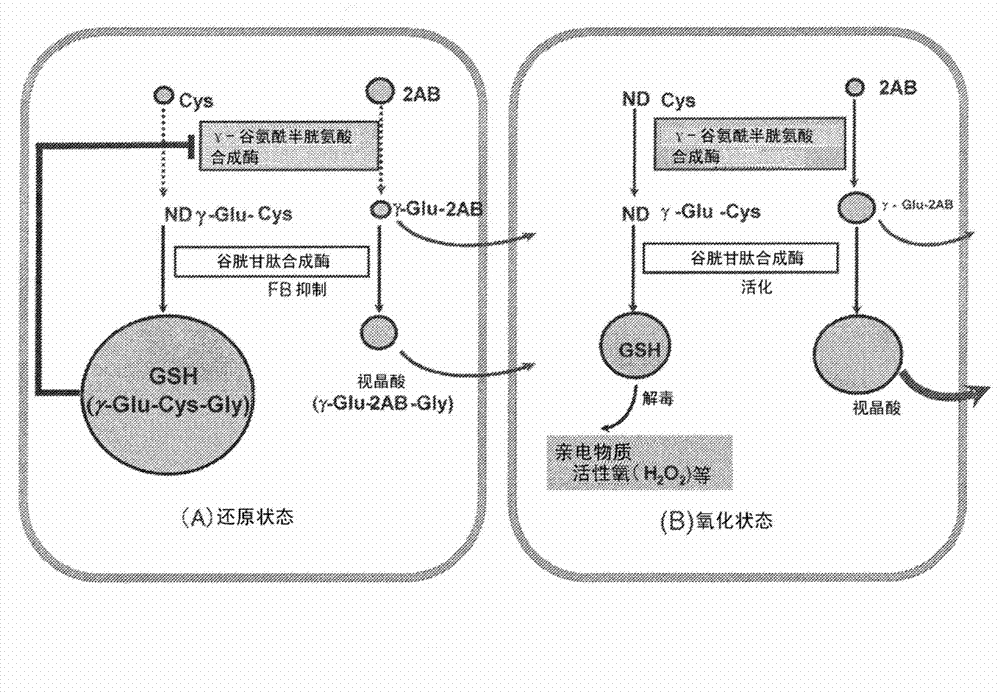
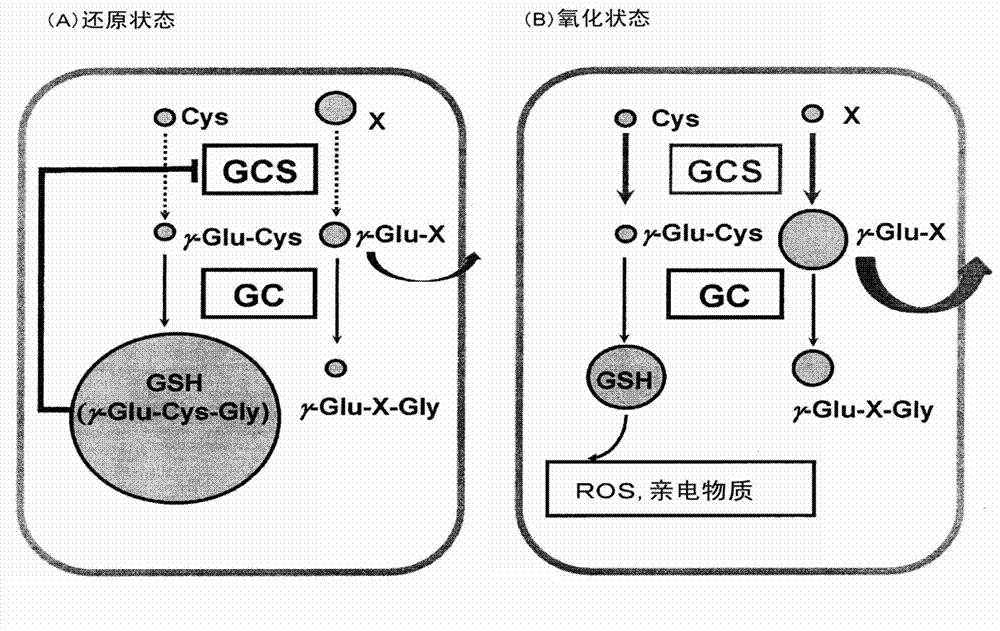
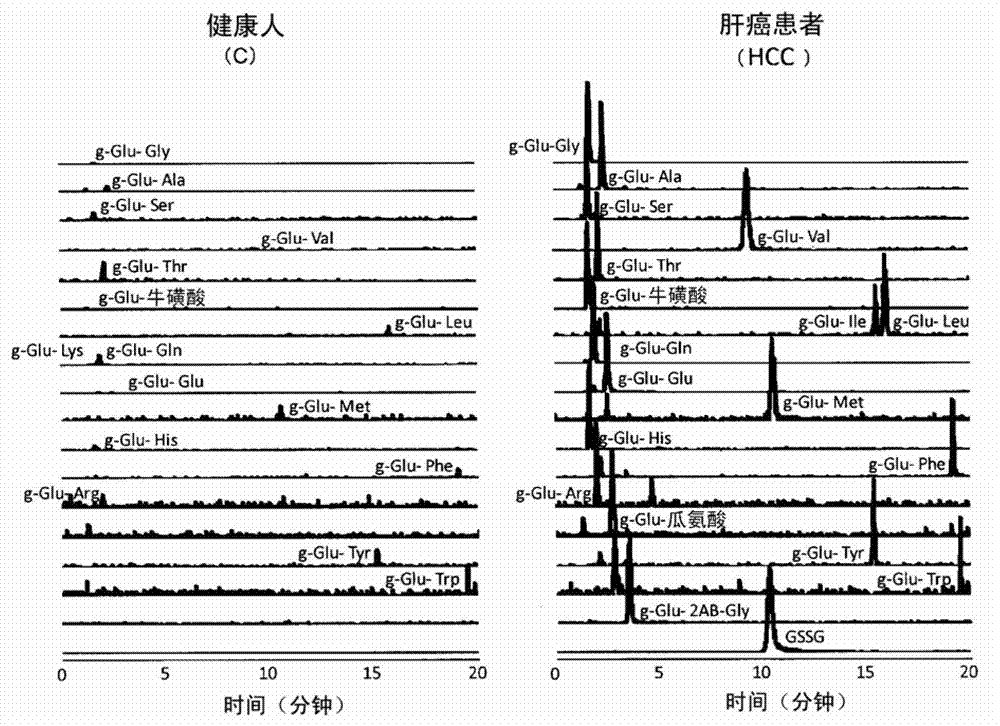
[0085] As described above, it is known that various liver injuries such as hepatitis, liver cirrhosis, and liver cancer are closely related to oxidative stress. Therefore, 53 healthy people (C), 10 drug-induced liver injury (DI), 9 asymptomatic hepatitis B carriers (AHB), 7 chronic hepatitis B (CHB), and 10 HCV positive ALT persistent Normal subjects (CNALT), 24 patients with chronic hepatitis C (CHC), 10 patients with liver cirrhosis (CIR), 19 patients with liver cancer (HCC), 11 patients with nonalcoholic steatohepatitis (NASH), 9 patients with simple fatty Liver (SS) sera were assayed for retinoic acid concentrations using capillary electrophoresis-time-of-flight mass spectrometry (CE-TOFMS). However, other substances were found to increase in predominance in each hepatitis patient, and they were all identified as γ-Glu-X peptides (Note: X indicates amino acid and amine).
[0086] 1. E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com