Compound amino acid injection (18AA-III) composition
A compound amino acid, injection technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of patient harm, unqualified antihypertensive substances, affecting product qualification rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
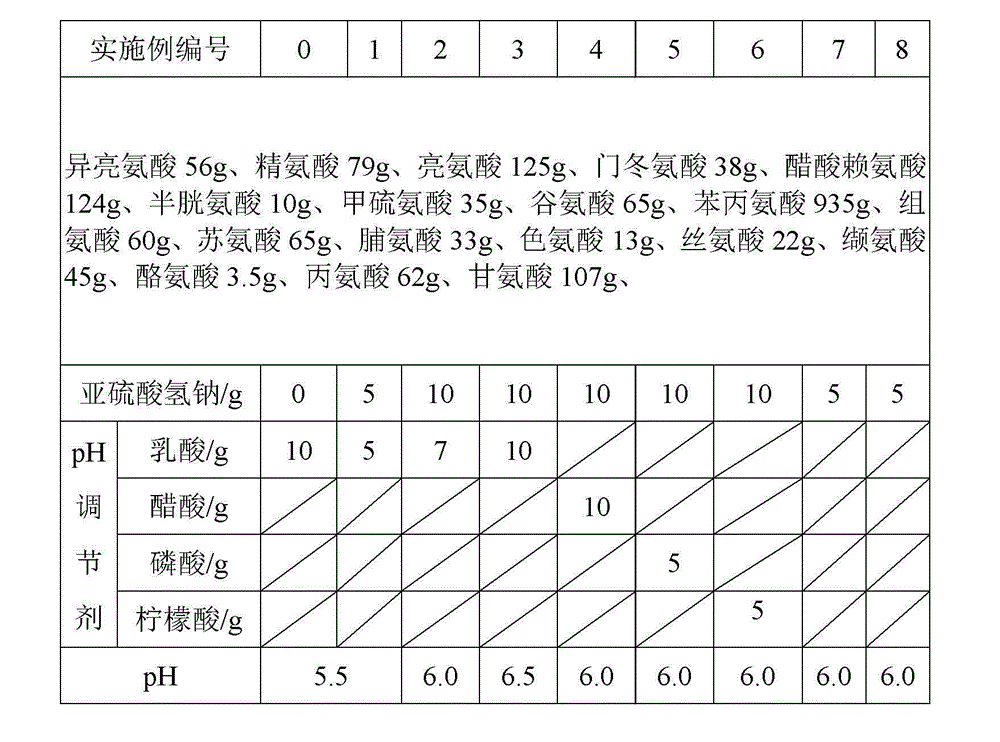
[0013] In the embodiment, the prescription in the following table is the feed amount calculated by 10000ml, and the subpackage adopts 250ml glass bottles. Sterilization was performed by autoclaving at 105°C for 45 minutes. After adding the pH regulator, adjust the pH to 5.5-6.5 with 0.1M sodium hydroxide solution or 0.1M hydrochloric acid. (If the pH is qualified, you don’t need to add more)
[0014] Described injection adopts following method to prepare
[0015] 1) Take 70% to 75% of the prescribed amount of water for injection, and add leucine, isoleucine, valine, methionine, phenylalanine, threonine, alanine, Proline, serine, aspartic acid, glutamic acid, arginine, lysine acetate, histidine, glycine and tryptophan make it fully soluble. Then add N-acetyl-L-tyrosine, fully stir cysteine hydrochloride until completely dissolved, add the prescribed amount of pH regulator (no pH regulator is added in Example 7-8), adjust with sodium hydroxide solution or hydrochloric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com