Method for generating hydrogen by hydrolyzing lithium borohydride and reacting device used for method
A lithium borohydride and reaction device technology, which is applied in the field of hydrogen source use of micro fuel cells, can solve problems such as slow reaction, reduce the hydrogen release rate, meet the output of low rate and high capacity, and promote the practical process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
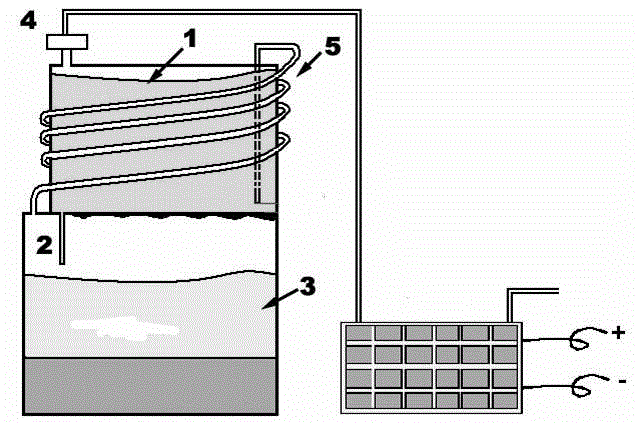
[0025] The reaction device used in this example is as figure 1 As shown, it includes a liquid water storage chamber 1, a water inlet capillary tube 2, a hydrolysis reaction chamber 3, a gas feedback pipeline 5, and an interface 4 opposite to the fuel cell. One end of the water inlet capillary tube 2 is in communication with the liquid water storage chamber 1 The other end is placed in the hydrolysis reaction chamber 3, one end of the gas feedback pipe 5 is inserted into the liquid water in the liquid water storage chamber 1, and the other end is in communication with the hydrolysis reaction chamber 3. The diameter of the water inlet capillary 2 is 5-30 μm.
[0026] 1g LiBH 4 Mix with 23 mL of ether solvent, that is, place it in the hydrolysis reaction chamber 3 of the reaction device according to the ratio of the saturated solution, and mechanically stir at 100 rpm until a uniform solution is formed. Then the reaction device is sealed and connected to the fuel cell through a micr...
Embodiment 2
[0028] The anyway device used in this embodiment is the same as that in embodiment 1.
[0029] 1g LiBH 4 Mix with 23 mL of ether solvent, that is, place it in the hydrolysis reaction chamber 3 of the reaction device according to the ratio of the saturated solution, and mechanically stir at 100 rpm until a homogeneous solution is formed, and then add 1 g of LiBH 4 For the solid powder, continue to stir for 2 hours, then seal the reaction device, communicate with the fuel cell, and inject excess liquid water into the liquid water storage chamber 1 through a micro pump. The liquid water drops into the hydrolysis reaction chamber 3 to react with the supernatant (saturated solution) of the supersaturated solution, and the resulting reaction product is deposited on the bottom of the reactor, and the consumed LiBH 4 LiBH at the bottom 4 The dissolved solids are constantly replenished. The rate of hydrogen produced by the system is 72mLmin -1 g -1 , Eventually LiBH 4 The hydrogen in it is...
Embodiment 3
[0031] The anyway device used in this embodiment is the same as that in embodiment 1.
[0032] 1g LiBH 4 Mix with 23 mL of ether solvent, that is, place it in the hydrolysis reaction chamber 3 of the reaction device according to the ratio of the saturated solution, and mechanically stir at 100 rpm until a uniform solution is formed, and then add 3 grams of LiBH 4 For the solid powder, continue to stir for 2 hours, then seal the reaction device, communicate with the fuel cell, and inject excess liquid water into the liquid water storage chamber 1 through a micro pump. The liquid water drops into the hydrolysis reaction chamber 3 to react with the supernatant (saturated solution) of the supersaturated solution, and the resulting reaction product is deposited on the bottom of the reactor, and the consumed LiBH 4 LiBH at the bottom 4 The dissolved solids are constantly replenished. The rate of hydrogen produced by the system is 160 mLmin -1 g -1 , Eventually LiBH 4 The hydrogen in it ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com