Glycine-L-cysteine-homotaurine tripeptide and application thereof
A technology of cysteine and homotaurine, applied in peptides, dipeptide components, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
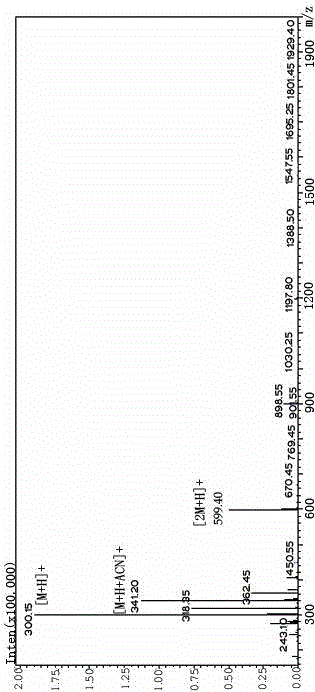
[0015] Glycine-L-cysteine-homotaurine tripeptide (Gly-Cys-NHCH 2 CH 2 CH 2 SO 3 H) The preparation includes the following steps: the final product Gly-Cys-NHCH 2 CH 2 CH 2 SO 3 H was synthesized by our company for the first time.
[0016] 1. Synthesis of N-Fmoc-S-trityl-L-cysteine-homotaurine [Fmoc-Cys(Trt)-NHCH 2 CH 2 CH 2 SO 3 H(Compound I)]
[0017] raw material molecular weight amount of substance Dosage N-Fmoc-S-trityl-L-cysteine (Fmoc-Cys(Trt)-OH) 585.73 12mmol 7g N-Hydroxysuccinimide (HOSu) 115.2 14mmol 1.6g Carbodiimide hydrochloride (EDCI) 191.7 14mmol 2.68g Homotaurine (NH 2 CH 2 CH 2 CH 2 SO 3 h) 139.17 10mmol 1.4g Na 2 CO 3 106 10mmol 1.06g
[0018] Fmoc-Cys(Trt)-OH (7 g) and HOSu (1.6 g) were dissolved in 40 ml tetrahydrofuran (THF), then EDCI (2.68 g) was added and stirred overnight at room temperature. Homotaurine (1.4g) and Na 2 CO 3 (1.06g) was dissolved in water (40ml...
Embodiment 2
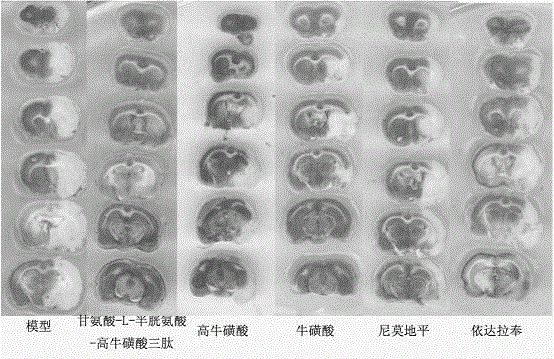
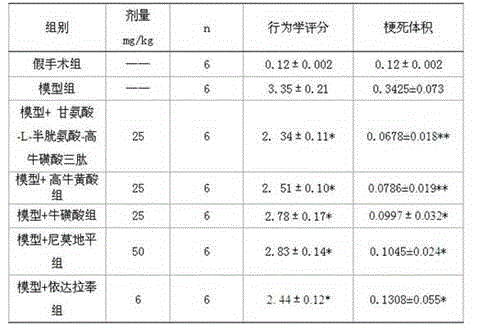
[0041] Glycine-L-cysteine-homotaurine tripeptide lipid microsphere injection, according to glycine-L-cysteine-homotaurine tripeptide 0.8%, lecithin for injection 2.3%, The injection is prepared by adding 6% of soybean oil, 6% of medium-chain fatty acid for injection, 2% of glycerin, 0.2% of Tween-80, 0.04% of sodium oleate and 100% of water for injection. Specific preparation method: disperse 2% glycerin for injection and 0.04% sodium oleate in appropriate amount of water for injection. Stir at 75°C until completely dissolved, and adjust the pH of the water phase to 6.5; add glycine-L-cysteine-homotaurine tripeptide to the mixed oil phase composed of soybean oil and medium-chain fatty acids, at 80°C Heat and stir for 30 minutes, then remove impurities through a 0.22 μm microporous membrane to obtain a clear primary oil phase, add phospholipids, heat and stir at 80°C, slowly add to the water phase, and stir for 10 minutes; the resulting colostrum is adjusted to pH with hydrochl...
Embodiment 3
[0043] The glycine-L-cysteine-homotaurine tripeptide composition comprises 100 g of glycine-L-cysteine-homotaurine tripeptide plus water for injection to 1 liter, and is packaged in 500 tubes. The preparation method is: take water for injection, add 0.5g of EDTA-2Na, add 100g of glycine-L-cysteine-homotaurine tripeptide to dissolve, add 24g of sodium bicarbonate powder in portions, stir until completely dissolved, and adjust the pH Between 5.8 and 6.2. Sterilized packaging, each containing glycine-L-cysteine-homotaurine tripeptide 200mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com