Interest protein preparation method and purpose thereof
A purpose and protein technology, applied in the field of target protein preparation, to achieve the effects of reducing difficulty, facilitating mass production, and saving cutting costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
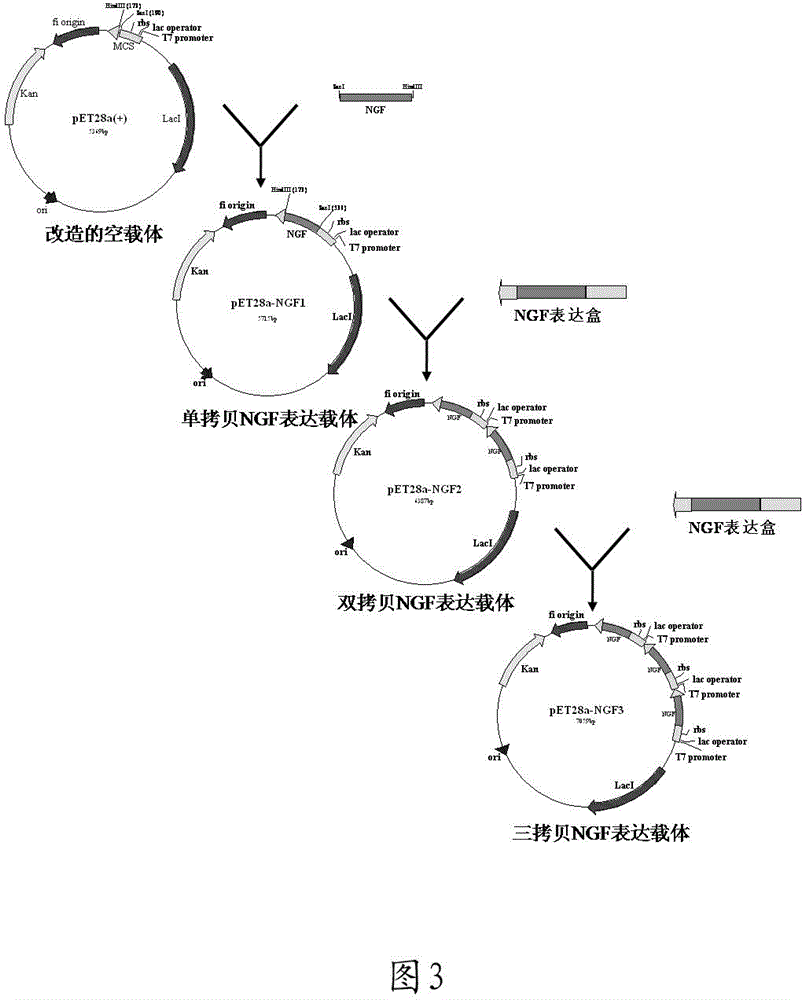
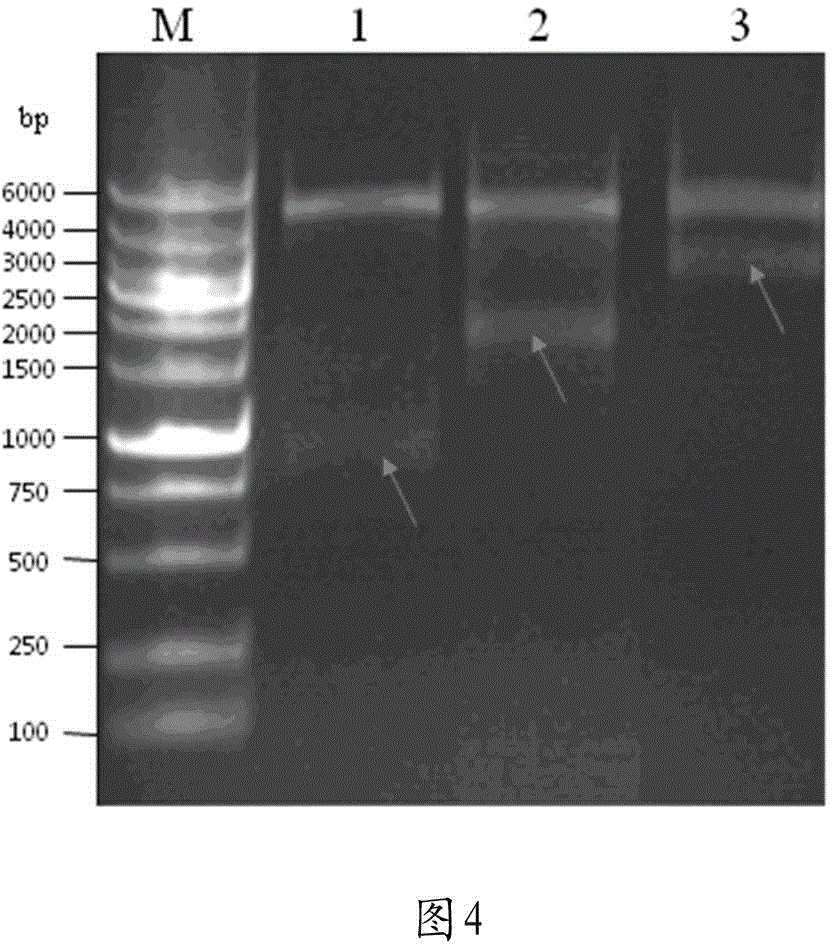
[0050] Example 1: High-efficiency expression of β-hNGF using the method of tandem multi-copy expression cassettes
[0051] 1. Transformation of prokaryotic expression plasmids
[0052] 1.1 Eliminate the BamHI-SalI-XhoI cutting point in the multiple cloning site (MCS) of the pET-28a (+) vector:
[0053] Synthesize the following oligonucleotide fragments:
[0054] pET28a-deBam-Xho-F:5'-GATCTGAATTCGAGCTCCTGCAGCAAGCTTGCGGCCGCA-3' (SEQ ID NO: 1)
[0055] pET28a-deBam-Xho-R:5'-TCGATGCGGCCGCAAGCTTGCTGCAGGAGCTCGAATTCA-3' (SEQ ID NO:2)
[0056] Denatured hybridization of pET28a-deBam-Xho-F and pET28a-deBam-Xho-R forms a hybrid fragment product, as shown in the following example:
[0057] 5'-GATCTGAATTCGAGCTCCTGCAGCAAGCTTGCGGCCGCA-3'
[0058] ||||||||||||||||||||||||||||||||||||
[0059] 3'-ACTTAAGCTCGAGGACGTCGTTCGAACGCCGGCGTAGCT-5'
[0060] The hybridization product was ligated with the recovered product of the pET-28a (+) vector fragment digested by BamHI / XhoI, and transf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com