Method for cleaner production of vitamin A intermediate product
A clean production and vitamin technology, applied in the field of pharmaceutical biochemical industry, can solve the problems of low conversion rate, long reaction cycle, environmental pollution and hidden dangers of operation safety, etc., and achieve the effect of high conversion rate, short production process and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
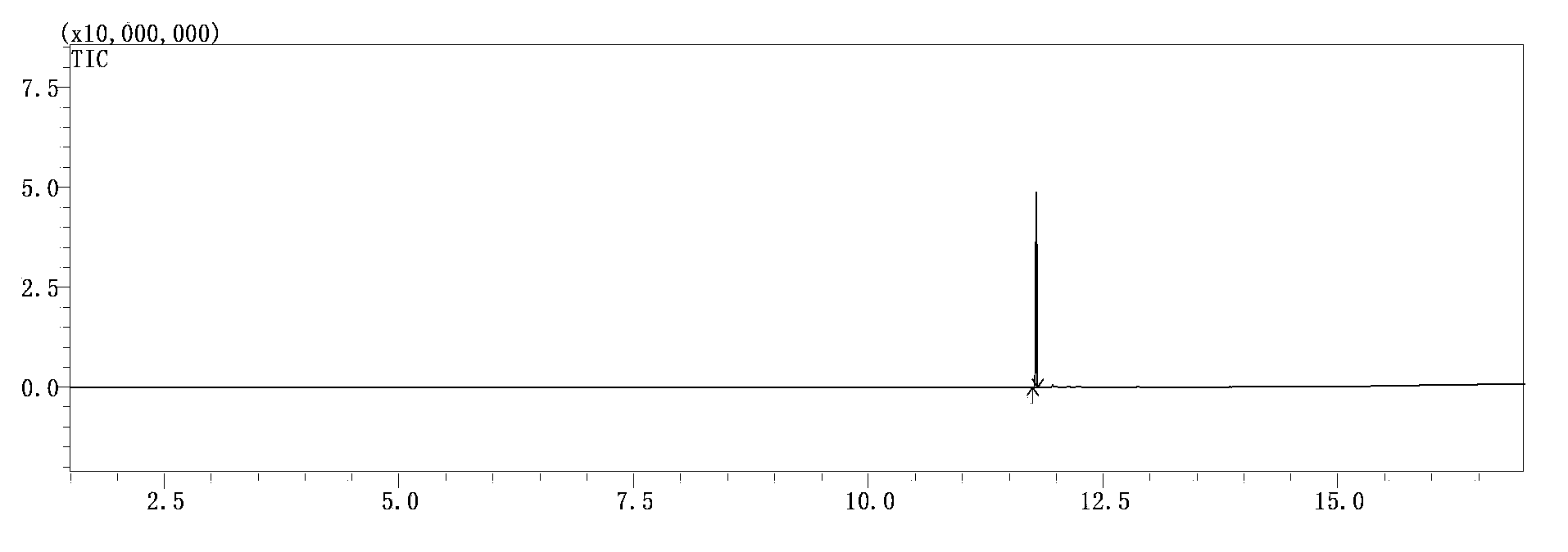
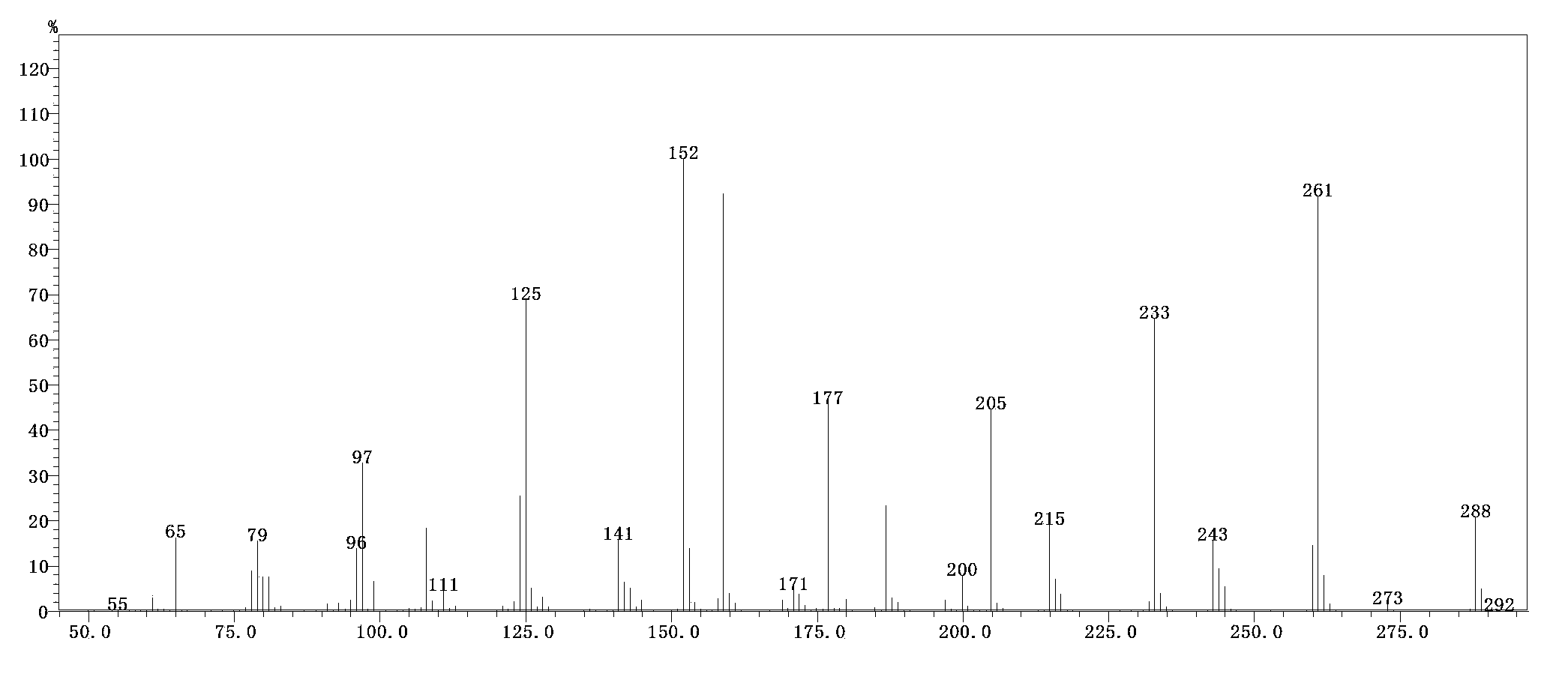
[0044] Add 276 grams of diethyl phosphite, 114 grams of diethoxymethane, 50 grams of absolute ethanol, and 2 grams of p-toluene sulfonic acid to a dry 1000 ml glass flask, and the temperature is raised to 75-80°C for 5 hours. GC detects that the phosphite diester reaction is complete, cool to 20°C, add 5 g of 28wt% sodium ethoxide ethanol solution to neutralize, distill at 180°C in a vacuum oil bath, collect the 130-136°C / 12 mbar fraction to obtain methylene 265 grams of tetraethyl bisphosphonate, GC purity 99.5%, yield 92.0%. The product has been compared with the standard product, the GC peak time is the same, and the GC-MS (gas chromatography-mass spectrometry detection) product ion current diagram is shown figure 1 ; And the product has been confirmed by GC-MS to confirm the molecular weight is correct, see the GC-MS mass spectrum figure 2 .
Embodiment 2
[0046] Add 276 grams of diethyl phosphite, 114 grams of diethoxymethane, and 2 grams of p-toluenesulfonic acid to a dry 1000 ml glass flask. The temperature is raised to 75-80°C and reacted for 5 hours. GC detects the diethyl phosphite. After the ester reaction is complete, cool to 20°C, add 5 g of 28wt% sodium ethoxide ethanol solution to neutralize, distill at 180°C in a vacuum oil bath, collect the 130-136°C / 12 mbar fraction to obtain methylene diphosphonic acid tetraethyl 259 grams of ester, GC purity 99.3%, yield 89.9%.
Embodiment 3
[0048] Add 276 grams of diethyl phosphite, 85 grams of dimethoxymethane, and 2 grams of p-toluene sulfonic acid to a dry 1000 ml reactor, gradually increase the temperature to 45-50°C and react for 2 hours, and the atmospheric pressure to 0.5 MPa, react at 85-90°C for 3 hours, GC detects that the phosphite diester reaction is complete, cool to 20°C, add 5 g of 28wt% sodium ethoxide ethanol solution for neutralization, distill at 180°C in a vacuum oil bath, collect 130-136°C / 12 mbar fraction, 261 grams of tetraethyl methylene diphosphonate was obtained, the purity was 99.1% by GC, and the yield was 90.6%.
[0049] Example 4-Example 12: The method steps are the same as in Example 1, except that different starting materials are used to prepare tetraethyl methylene diphosphonate or tetramethyl methylene diphosphonate under different conditions , The specific raw material ratio and reaction conditions, solvent, catalyst, neutralizer, product name, GC purity, product quality and yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com