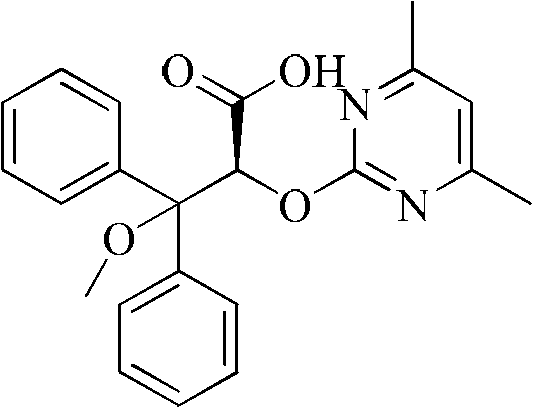
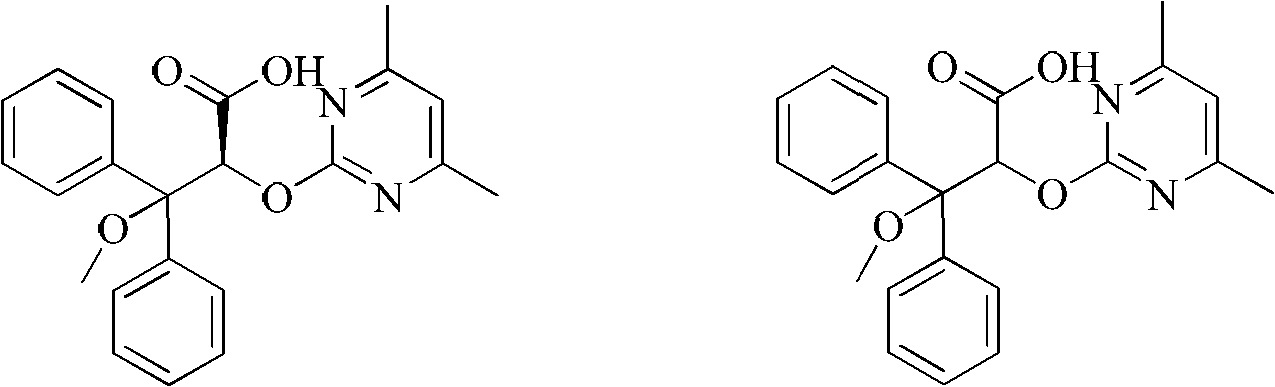
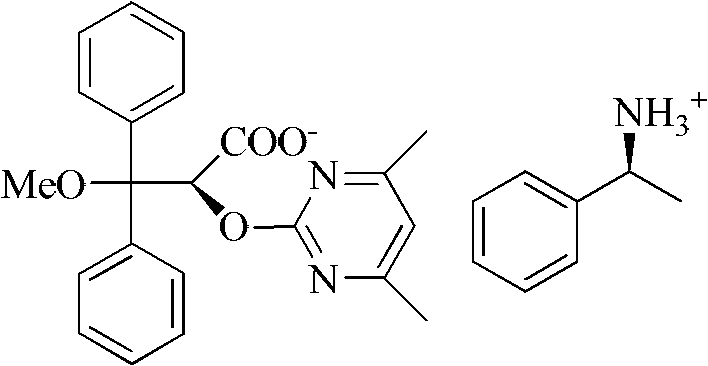
Method for preparing ambrisentan
An ambrisentan and solvent technology, applied in the field of chemistry, can solve the problems of potential safety hazards in the use of lithium amide, unsuitable for industrial production, expensive resolving agents, etc., and achieves the effects of good separation efficiency, easy recovery, and improved safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In order to enable those skilled in the art to better understand the technical solutions of the present invention, the present invention will be further described below in conjunction with examples, but these examples do not constitute any limitation to the present invention. 1 HNMR was recorded by AM 400 nuclear magnetic resonance instrument, and chemical shifts were expressed in δ (ppm). The mass spectrum was measured with a Shimadzu LCMS-2010 mass spectrometer, and the optical rotation was measured with a Perkin-Elmer 341 polarimeter. Embodiment 1: the preparation of ambrisentan
[0026] Take a 50ml one-mouth bottle, add 3.04g (8mmol) 2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropionic acid, 0.97g (8mmol) (S)-phenethylamine, 30ml methanol. After reflux reaction for 2 hours, it was lowered to room temperature, methanol was distilled off, 20 ml of isopropanol was added for recrystallization, cooled to room temperature naturally, and 1.40 g of solid wa...
Embodiment 2
[0035] Embodiment 2: the preparation of ambrisentan
[0036] Take a 50ml one-mouth bottle, add 3.04g (8mmol) 2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropionic acid, 0.97g (8mmol) (S)-phenethylamine, 30ml methanol. After reflux reaction for 2 hours, it was lowered to room temperature, methanol was distilled off, 15 ml of ethyl acetate was added for recrystallization, cooled to room temperature naturally, and 1.28 g of solid was obtained by filtration.
[0037] The solid was added with 30ml of water, 30ml of ethyl acetate, dilute hydrochloric acid to adjust the pH to acidic, and the organic layer was separated. The organic layer was washed with water, dried, and concentrated to obtain 0.8g of a white solid, with a yield of 26%.
[0038] [a] D 20 =+180.3° (C=0.5, MeOH)
Embodiment 3
[0039] Embodiment 3: the preparation of ambrisentan
[0040] Take a 50ml one-mouth bottle, add 3.04g (8mmol) 2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropionic acid, 1.46g (12mmol) (S)-phenethylamine, 30ml ethanol. After reflux reaction for 2 hours, it was cooled to room temperature, ethanol was distilled off, 15ml of isopropanol was added for recrystallization, cooled to 0°C, and 1.45g of solid was obtained by filtration.
[0041] The solid was added with 30ml of water, 30ml of ethyl acetate, dilute hydrochloric acid to adjust the pH to acidic, and the organic layer was separated. The organic layer was washed with water, dried and concentrated to give 1.0g of white solid with a yield of 33%.
[0042] [a] D 20 =+100.3° (C=0.5, MeOH)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com