A kind of preparation method of chiral phenyl lactic acid
A technology of phenyllactic acid and phenyllactate, which is applied in the preparation of carboxylate, the preparation of organic compounds, organic chemical methods, etc., can solve the problem of no biosynthesis method, difficulty in processing industrial waste, environmental pollution of dangerous processes, etc. problem, to achieve the effect of good splitting efficiency, cheap recycling, and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
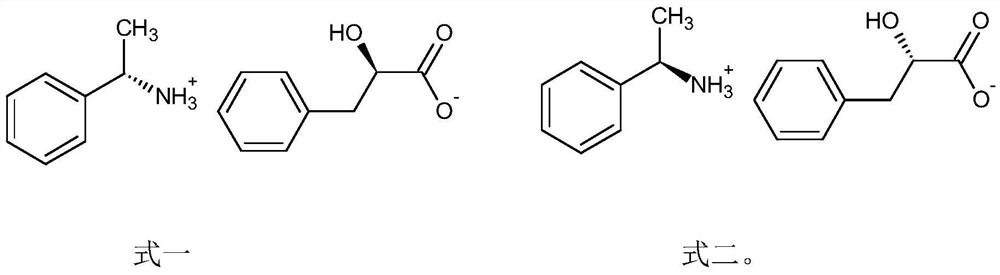
[0021] Synthesis of (S)-Phenylethylamine-D-Phenyl Lactate
[0022] Add 3.3 g of DL-phenyl lactic acid, 30 ml of ethyl acetate / methanol (volume ratio 2:1) mixture, add 1.5 g of (S)-phenylethylamine, heat properly until completely dissolved, cool to below room temperature, A solid was precipitated to obtain 1.5 g of a crude product of (S)-phenethylamine-D-phenyllactate with an optical rotation of +24° (C=1 ethanol).
Embodiment 2
[0024] Synthesis of (S)-Phenylethylamine-D-Phenyl Lactate
[0025] Add 3.3 g of DL-phenyllactic acid, 30 ml of ethyl acetate / ethanol (volume ratio 3:1) mixture, add 2.0 g of (S)-phenylethylamine, heat properly until completely dissolved, then cool to below room temperature, A solid was precipitated to obtain 1.6 g of a crude product of (S)-phenethylamine-D-phenyllactate with an optical rotation of +28° (C=1 ethanol).
Embodiment 3
[0027] Synthesis of (S)-Phenylethylamine-D-Phenyl Lactate
[0028] Add 3.3 g of DL-phenyl lactic acid, 20 ml of ethyl acetate / ethanol (volume ratio 4:1) mixture, add 2.4 g of (S)-phenylethylamine, heat properly until completely dissolved, then cool to below room temperature, A solid was precipitated to obtain 1.7 g of a crude product of (S)-phenethylamine-D-phenyllactate with an optical rotation of +30° (C=1 ethanol).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com