A kind of preparation method of ruxolitinib phosphate
A technology of arus phosphate and tinib, applied in the field of medicine, can solve the problems of low chiral purity, high cost of precious metal catalysts, unsuitable for scale-up production, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (Compound 2)
[0056] Dissolve some 4-chloro-7H-pyrrolo[2,3-d]pyrimidine in 3 volumes of N,N-diethylphenylacetamide, protect with nitrogen, lower the temperature below -10°C, and add sodium hydride in batches (1.05eq), after adding, raise the temperature to 20~25°C, stir for 1~2h, then control the temperature below -5°C, add dry 2-(trimethylsilyl)ethoxymethyl chloride dropwise (1.05eq), after dropping, raise the temperature to 20-25°C, react for 1-2h, monitor by TLC until the reaction is completed, add 6 times the volume of saturated ammonium chloride solution to quench the reaction. The precipitated solid was filtered, and the filter cake was washed with water and tertiary methyl ether. Blast drying at 40~45°C to obtain 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine in a yield of 95 %, purity ≥ 94.0%. used directly in the next reaction.
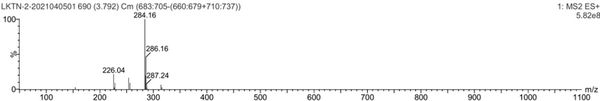
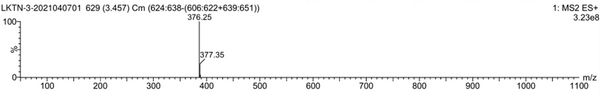
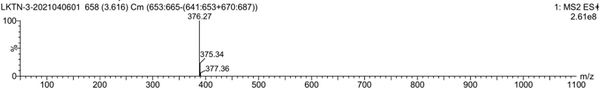
[0057] Mass ...
Embodiment 2
[0059] Preparation of 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (compound 2)
[0060] Dissolve some 4-chloro-7H-pyrrolo[2,3-d]pyrimidine in 4 volumes of N,N-diethylphenylacetamide, protect with nitrogen, lower the temperature below -10°C, and add sodium hydride in batches (1.05eq), after adding, raise the temperature to 20~25°C, stir for 1~2h, then control the temperature below -5°C, add dry 2-(trimethylsilyl)ethoxymethyl chloride dropwise (1.0eq), after dropping, raise the temperature to 20-25°C, react for 1-2h, monitor by TLC until the reaction is completed, add 6 times the volume of saturated ammonium chloride solution to quench the reaction. The precipitated solid was filtered, and the filter cake was washed with water and tertiary methyl ether. Blast drying at 40~45°C to obtain 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine in a yield of 91 %, purity ≥ 91.0%. used directly in the next reaction.
Embodiment 3
[0062] 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-7-((2-(trimethylsilyl)ethoxy)methyl base)-7H-pyrrolo[2,3-d]pyrimidine (compound 3)
[0063] Dissolve some 4-chloro-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine in 4 volumes of dimethyl sulfoxide , under nitrogen protection, lower the temperature to below -10°C, add isopropylmagnesium chloride (1.05eq) dropwise, after the addition, raise the temperature to 0-5°C, stir for 1-2 hours, then control the temperature below 0-5°C, add After the addition of dry pinacol ester borate (1.05eq), react at 0-5°C for 1-2h, monitor by TLC until the reaction is complete, add 6 times the volume of saturated ammonium chloride solution to quench the reaction. Add 6 times the volume of ethyl acetate to the feed solution for extraction, wash with water, wash with saturated brine, dry over anhydrous Na2SO4, filter, and concentrate under reduced pressure to obtain 4-(4,4,5,5-tetramethyl-1,3,2 -Dioxaborolan-2-yl)-7-((2-(trimet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com