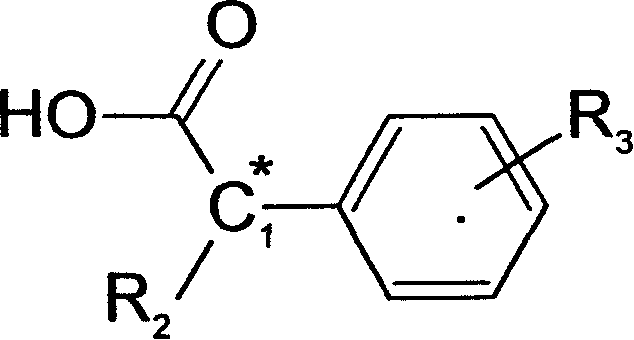
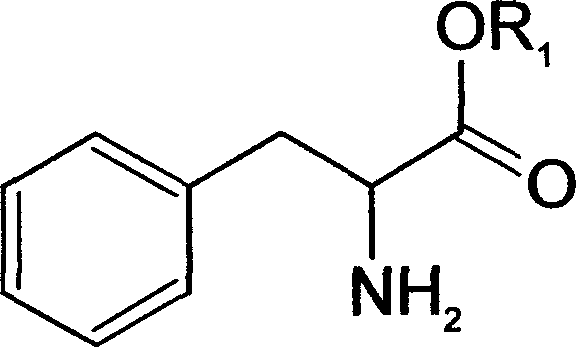
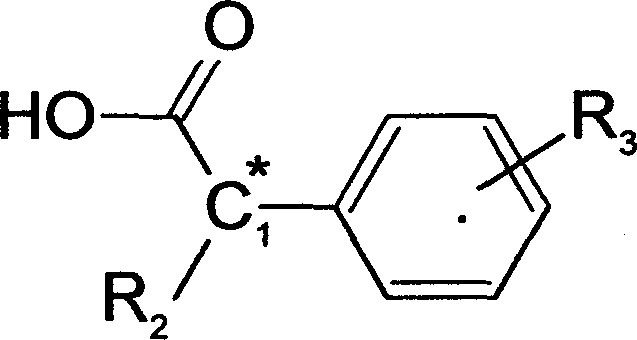
Method of chiral separation for D,L-phenylalanine ester or its salt
A phenylalanine ester, chiral resolution technology, applied in the preparation of D-type or L-type phenylalanine ester, the preparation of other chiral amino acid ester compounds, the preparation of chiral organic compounds, to reduce the The effect of production cost, low price and efficient splitting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 18.5 grams (0.1mol) of D-alpha-methyl p-chlorophenylacetic acid and D, 17.9 grams (0.1mol) of L-phenylalanine methyl ester, in the water content of 200ml, be dissolved in the water-ethanol solution of 80%, The temperature was raised to 75°C to make the solution clear, and then the temperature was slowly lowered to 4°C to allow it to fully crystallize for 8 hours. The resulting white crystals were filtered, which was the crude product of D-α-methyl-p-chlorophenylacetic acid·L-phenylalanine methyl ester, and washed with 50% ethanol solution. This substance was recrystallized in an 80% water-ethanol solution to obtain 16.5 grams of refined salt of D-α-methyl p-chlorophenylacetic acid·L-phenylalanine methyl ester.
[0046] Dissolve 15 grams of D-α-methyl p-chlorophenylacetic acid·L-phenylalanine methyl ester refined salt in 100 ml of water-ethanol solution with a water content of 80%, and adjust the pH value to 2 with 3N hydrochloric acid solution. Ethanol was distilled of...
Embodiment 2
[0048] 18.5 g (0.1 mol) of L-α-methyl p-chlorophenylacetic acid and 17.9 g (0.1 mol) of partially racemic D, L-phenylalanine methyl ester, the optical purity (% o.p.) is expressed as D-benzene Alanine methyl ester is calculated as 65%, dissolved in 150ml of methanol solution, heated to 70°C to make the solution clear, and then slowly cooled to 4°C to allow it to fully crystallize for 8 hours. The resulting white crystals were filtered, which was the crude product of L-α-methyl-p-chlorophenylacetic acid·D-phenylalanine methyl ester, and washed with 50% methanol solution. This substance was recrystallized in a water-methanol solution with a water content of 80%, to obtain 26.3 grams of refined salt of L-α-methyl p-chlorophenylacetic acid·D-phenylalanine methyl ester.
[0049] Dissolve 15 grams of L-α-methyl p-chlorophenylacetic acid·D-phenylalanine methyl ester refined salt in 80 ml of methanol solution, and then add 15 ml of 3N sulfuric acid solution. And cooling down to 4°C, ...
Embodiment 3
[0051] According to the method of Example 1, D, L-phenylalanine ethyl ester can be salified with D-α-methyl p-chlorophenylacetic acid to form white D-α-methyl p-chlorophenylacetic acid L-phenylalanine Ethyl double salt. Then the method for processing double salt by embodiment 1 can obtain L-phenylalanine ethyl ester hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com