Improvement of expression quantity of foreign proteins by fusion label
A technology that fuses tags and proteins, and is applied in the introduction of foreign genetic material using vectors, microorganism-based methods, microorganisms, etc., and can solve the problems of low expression and failure to meet the requirements of industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
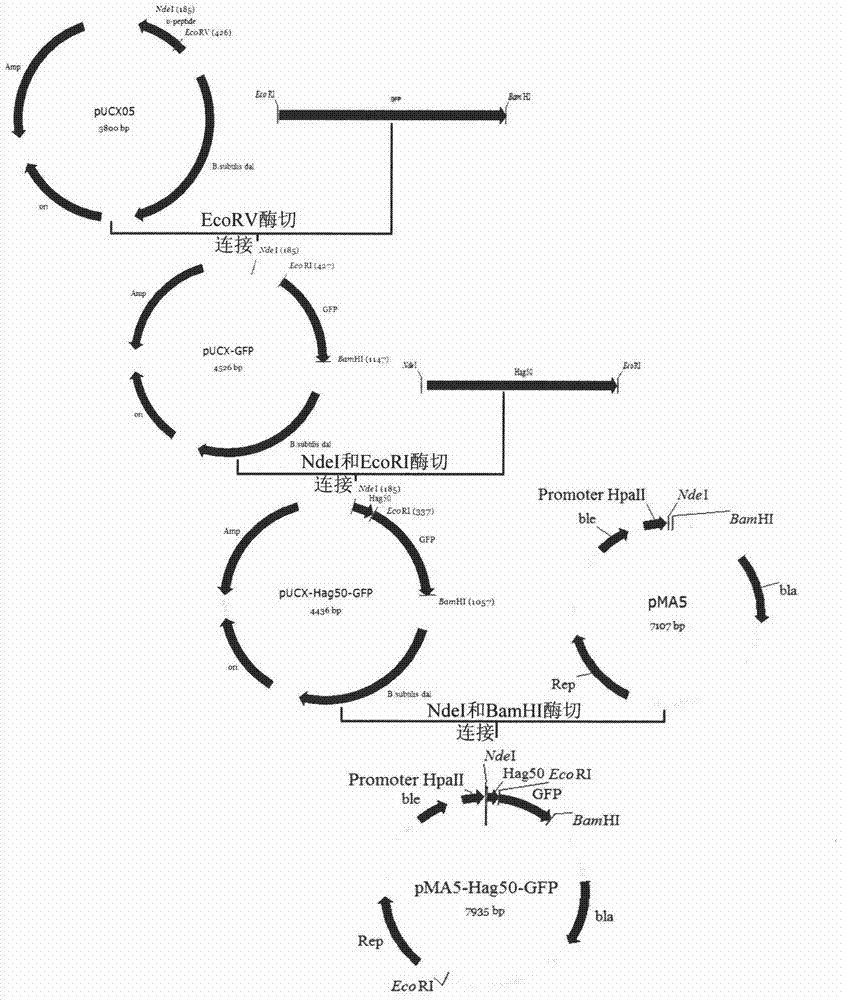
[0016] 1. Obtaining the first 50 amino acid coding sequences of Bacillus subtilis flagellin Hag
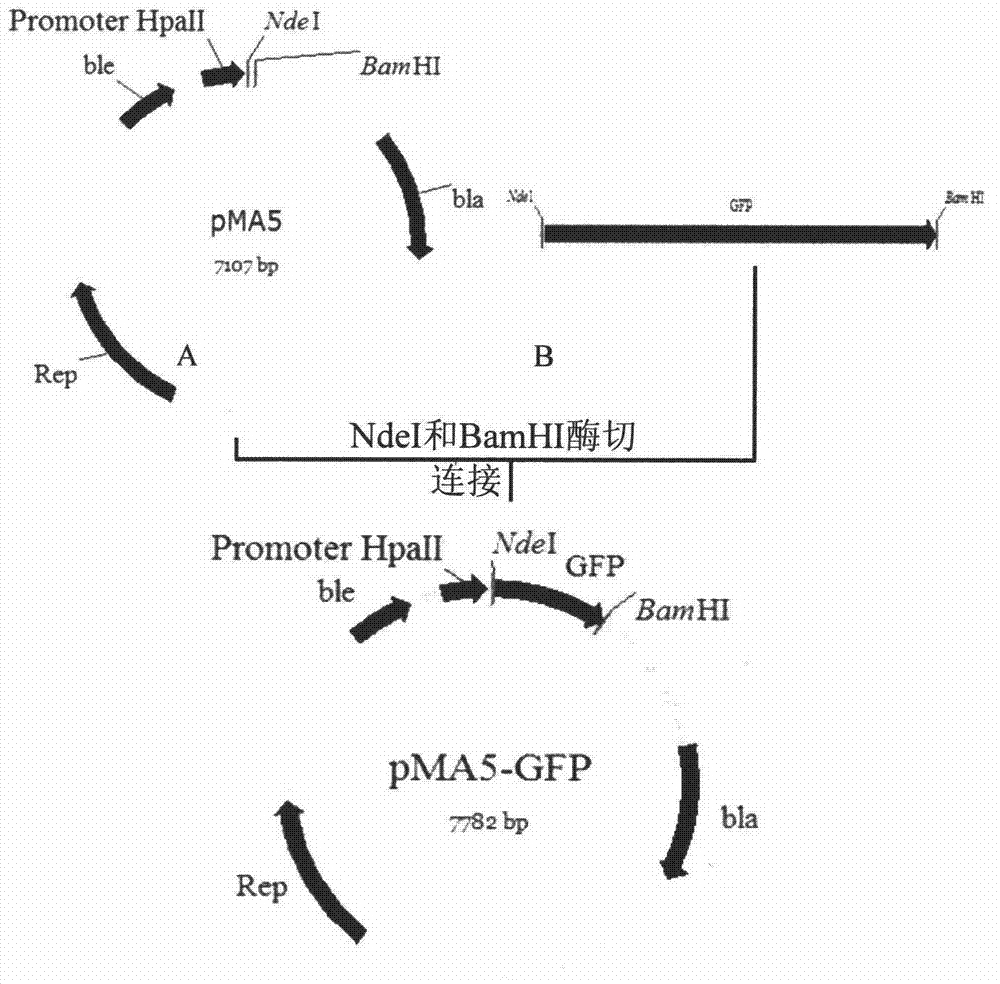
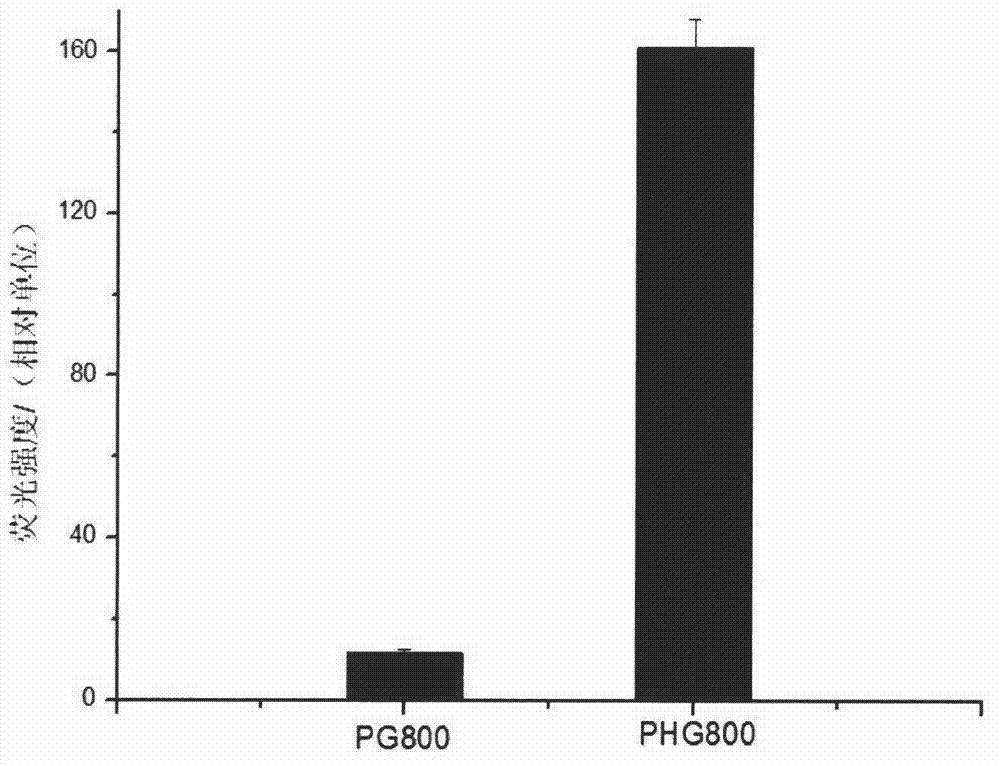
[0017] The hag gene of B. subtilis 168 (Genbank sequence number: M26948) is 915 bp in full length, and is one of the most expressed proteins in Bacillus subtilis. Genomic DNA was extracted from the cultured B. subtilis168 bacterial liquid according to the instructions of the Bacterial Genome Rapid Extraction Kit. PCR primers hag-F and hag50-R were designed according to the hag gene sequence in the whole genome of B.subtilis168 published by NCBI, and NdeI and EcoRI restriction sites were introduced into the upstream and downstream primers respectively. The primers were provided by Sangon Bioengineering (Shanghai) Limited Company Synthesis. Using the extracted genomic DNA as a template, the first 50 amino acid coding sequences of flagellin Hag were amplified by PCR. The amplified product was detected by agarose gel electrophoresis, and the size was consistent with the expectation. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com