A kind of preparation method of amorphous tofacitinib citrate
A technology of tofacitinib and citric acid, which is applied in the field of pharmacy, can solve the problems of cumbersome operation, many impurities in the product, and reduced product purity and quality, and achieve the effects of simple preparation method, good stability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
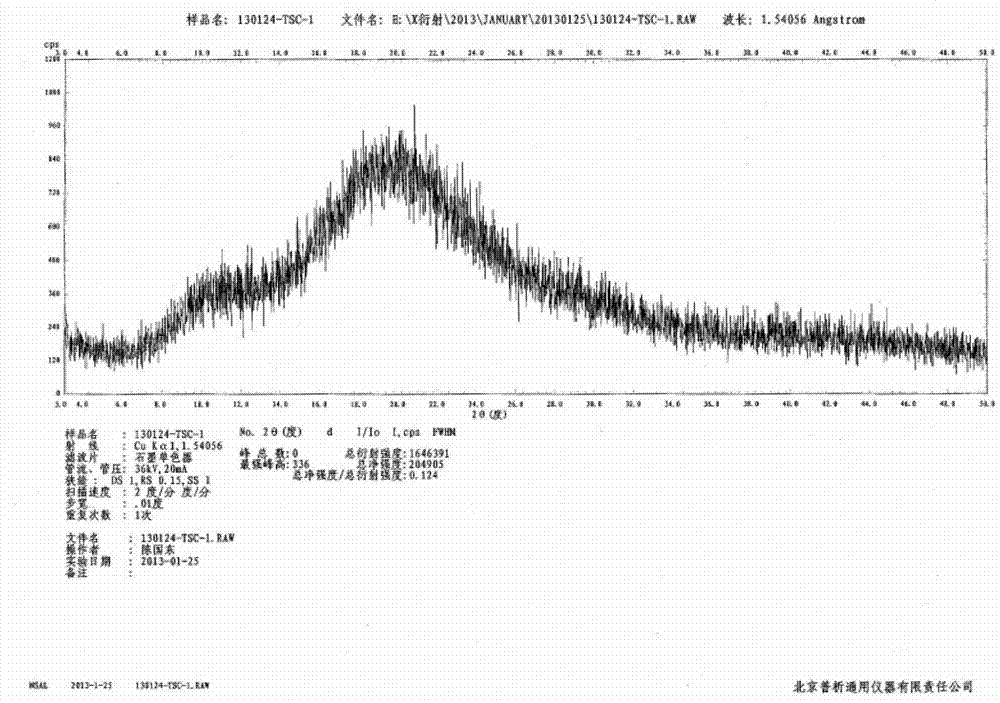
[0027] Example 1: Preparation of amorphous tofacitinib citrate (ethanol-water system) by precipitation method
[0028] Add 5g of tofacitinib citrate into the reaction flask, add 20mL of ethanol, heat to 30-35°C to completely dissolve the solid, add the solution to 20mL of 15-25°C water, and stir at 15-25°C The suspension was filtered for about 16 hours, the obtained solid was washed with ethanol / water (volume ratio 1:1), and air-dried at 60°C to constant weight to obtain 4.5 g of amorphous tofacitinib citrate with a yield of 95 %, HPLC analysis purity is 99.6%.
Embodiment 2
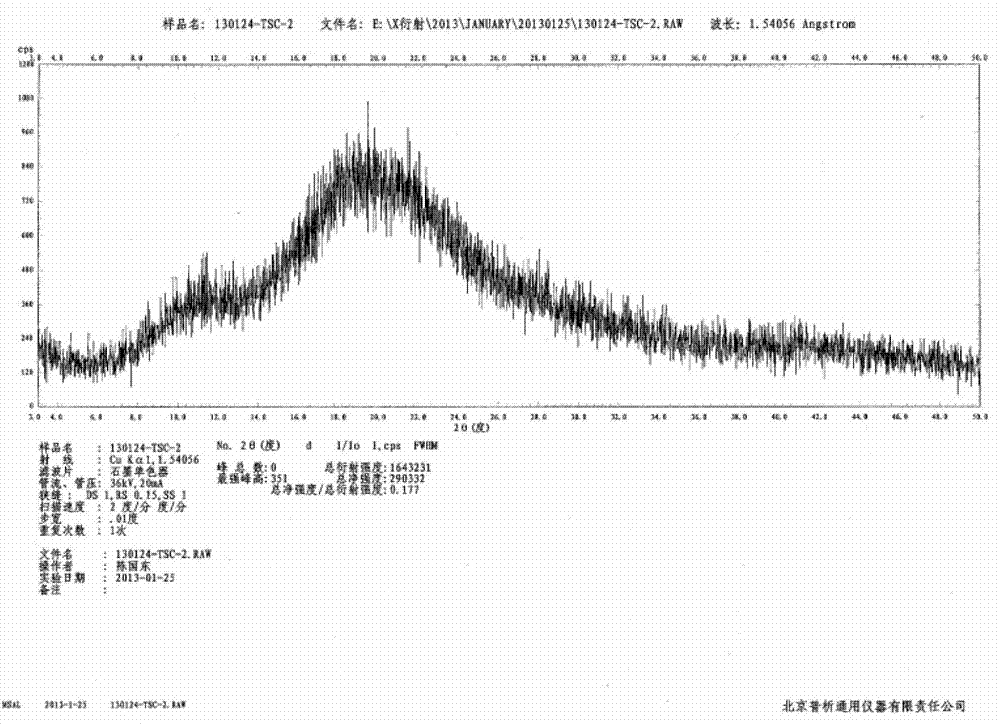
[0029] Example 2: Preparation of amorphous tofacitinib citrate (methanol-ethyl acetate system) by precipitation method
[0030] Add 50g of tofacitinib citrate into the reaction flask, add 100mL of methanol, heat to 30-35°C to completely dissolve the solid, add the solution to 50mL of ethyl acetate at 25-30°C, and heat at 25-30°C The resulting suspension was stirred at low temperature for about 20 hours, filtered, the obtained solid was washed with methanol / ethyl acetate (volume ratio 2:1), and air-dried at 60°C to constant weight to obtain 45.8 g of amorphous tofacitinib citrate , the yield was 91.6%, and the HPLC analysis purity was 99.7%.
Embodiment 3
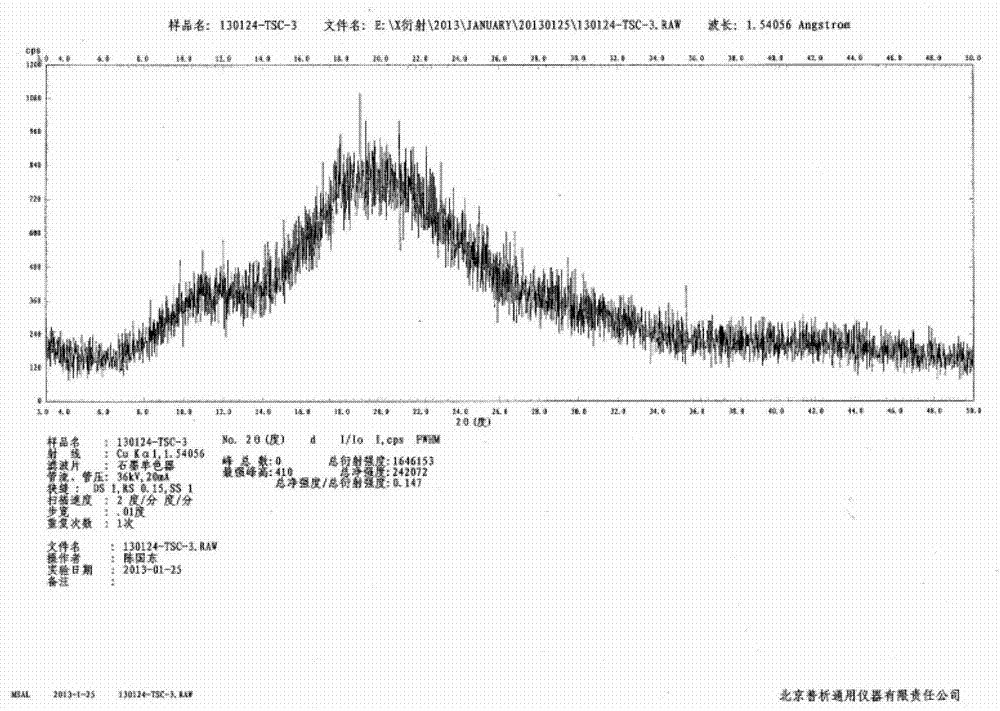
[0031] Embodiment 3: Preparation of amorphous tofacitinib citrate by spray drying method
[0032] 50 g of tofacitinib citrate was dissolved in 100 mL of methanol at about 30-35° C., and the solution was spray-dried at a flow rate of about 2.5 ml / min at 30° C. to 35° C. in a nitrogen flow of 600 Nl / h. The substance was recovered from the receiver, and the obtained solid was air-dried at 60°C to constant weight to obtain 46.8 g of amorphous tofacitinib citrate, with a yield of 93.6% and a purity of 99.5% by HPLC analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com