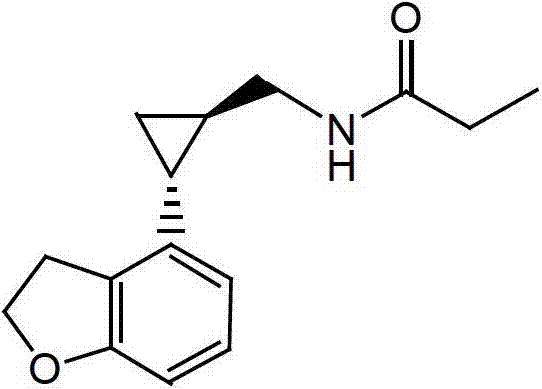
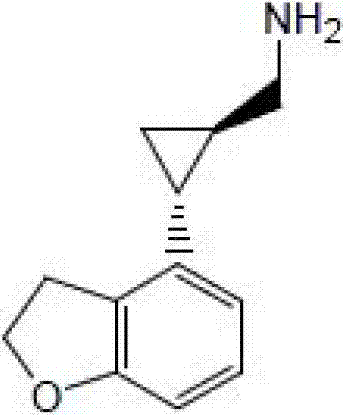
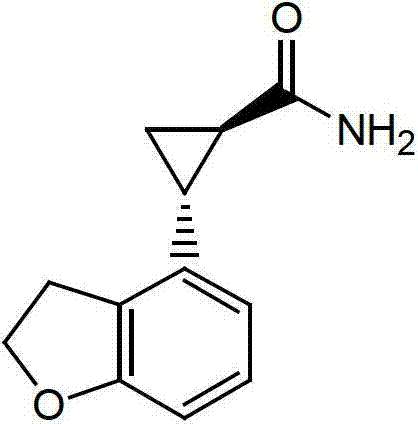
Preparation method of tasimelteon
A technology of tasimelteon and reducing agent, which is applied in the field of preparation of tasimelteon, can solve the problems of complexity, economical reduction, and long reaction steps, and achieve the effects of reducing reaction steps, improving utilization rate, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 2.0g of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanecarboxamide and 1.9g of sodium borohydride to 40ml of anhydrous tetrahydrofuran and heat to reflux, where ( The molar ratio of 1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanamide to sodium borohydride is about 1:5. Add 12.5g propionic acid and 20ml anhydrous slowly. The mixed solution of tetrahydrofuran (the molar ratio of sodium borohydride to propionic acid is about 1:3.3), add it in 12 hours, then continue the reaction at 70°C for about 6 hours, and then drop to room temperature. Pour the materials slowly into 100ml ice water and stir. Add dropwise about 20g of hydrochloric acid with a mass concentration of 10%, and adjust the pH to about 2. The obtained product was distilled under reduced pressure to remove the solvent tetrahydrofuran, and then extracted with 60 ml of dichloromethane. The aqueous phase was extracted once with 30 ml of dichloromethane. The organic phases were combined. The obtained organi...
Embodiment 2
[0036] In addition to adding 0.78g sodium borohydride and 2.8g propionic acid, the molar ratio of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanamide to sodium borohydride is about The molar ratio of sodium borohydride to propionic acid is about 1:1.8. Tasmetron was prepared according to the same process as in Example 1, to obtain 1.79 g of white needle-shaped crystals of tasmetron with a purity of 98.7%. The rate is 85%.
Embodiment 3
[0038] In addition to adding 3.51g sodium borohydride and 35.1g propionic acid, the molar ratio of (1R,2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropanamide to sodium borohydride is about The molar ratio of sodium borohydride to propionic acid is about 1:5. Tasmetron was prepared according to the same process as in Example 1, to obtain 2.3 g of white needle-shaped crystals of tasmetron with a purity of 99.9%. The rate is 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com