Application of N-acetylglucosamine isomerase in production of N-acetylmannosamine
A technology of acetylglucosamine and acetylmannosamine, which is applied in the field of invention, can solve problems such as undiscovered PmAGE, and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
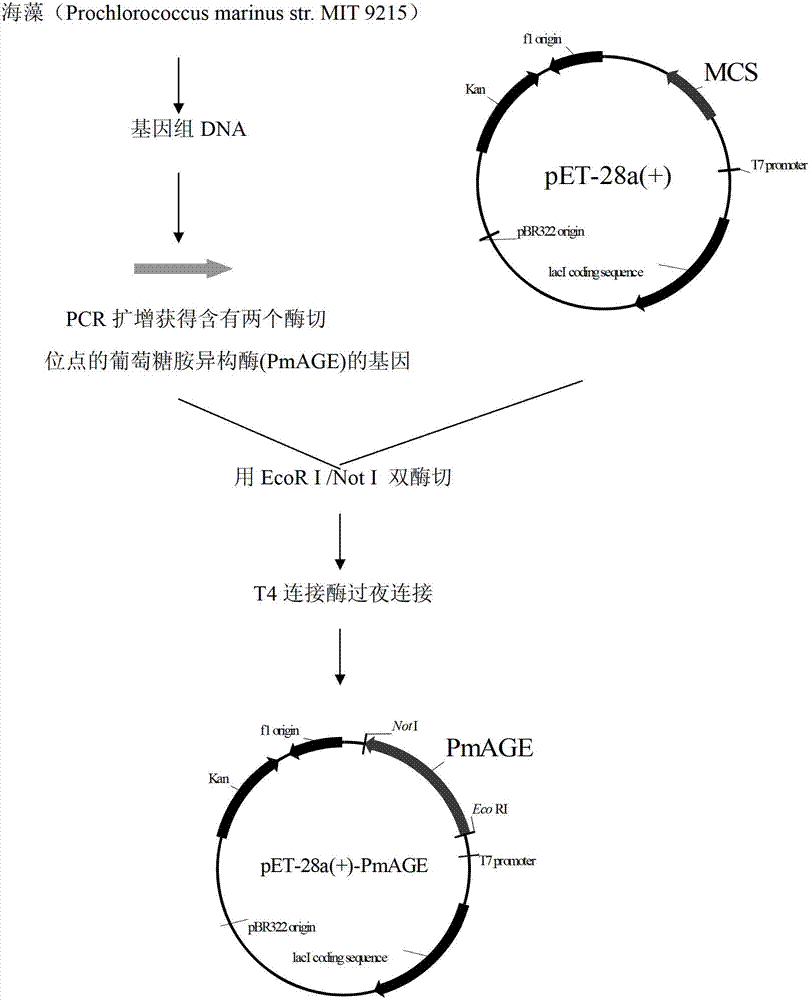
[0033] Example 1: Construction of recombinant Escherichia coli E. coli Rosseta (pET28a-PmAGE).
[0034] 1. Acquisition of N-acetylglucosamine isomerase gene:
[0035] Cells of marine-derived cyanobacteria Prochlorococcus marinus str. MIT9215 (from Prof. Chisholm, Massachusetts Institute of Technology) were frozen in liquid nitrogen for 1 minute and then transferred to 100°C boiling water for 10 minutes. The seaweed thus treated was used as a template for PCR.
[0036] The primers used to construct the expression vectors are provided with enzyme cutting sites, and the primer sequences are as follows:
[0037] The upstream primer (PmAGE-sense containing EcoR I) is:
[0038] 5'CCG GAATTC ATGCAAAAATATATAAACGAATATCTAAG
[0039] Downstream primers (PmAGE-anti with Not I) are:
[0040] ATTT GCGGCCGC TTATTTAAACAATGTTGTATTTTTT
[0041] All primers were synthesized by Nanjing GenScript Company.
[0042] Gene PCR conditions:
[0043] Denaturation at 94°C for 5 min, cycled 30 t...
Embodiment 2
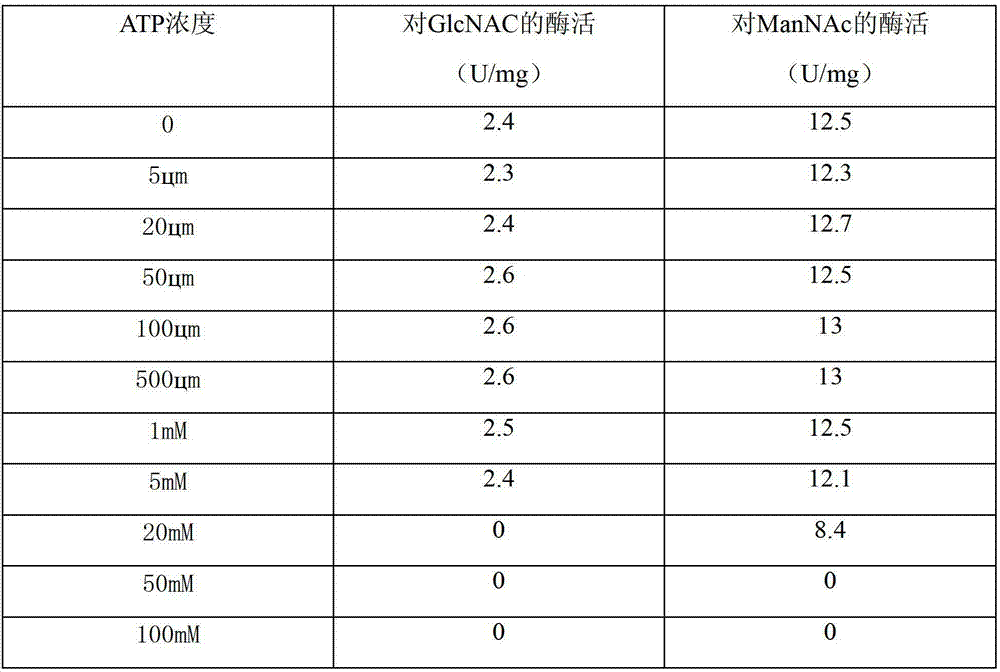
[0046] Example 2: Study on the acquisition and properties of the isomerase PmAGE.
[0047] 1. Expression of N-acetylglucosamine isomerase PmAGE.
[0048] Pick the recombinant strain E.coli Rosseta (pET-28a-PmAGE) into the LB liquid medium containing antibiotics, and cultivate it overnight at 37°C with shaking. Then they were inoculated into fresh culture medium according to 2% inoculation amount, and cultivated to OD at 37°C. 600 At about 0.6, add IPTG to a final concentration of 0.5mmol·L -1 , 15°C, 220rpm, after induction of expression for 12h, centrifuge (4°C, 10000rpm, 10min).
[0049] 2. Purification of N-acetylglucosamine isomerase PmAGE.
[0050] The collected sludge was resuspended with phosphate buffer (50mM, pH7.5) containing 300mM NaCl and 30% glycerol, and the cells were disrupted by ultrasonic (power 300W, ultrasonic 5s, intermittent 5s, total 5min), centrifuged (4°C, 12000rpm, 15min) to take the supernatant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com