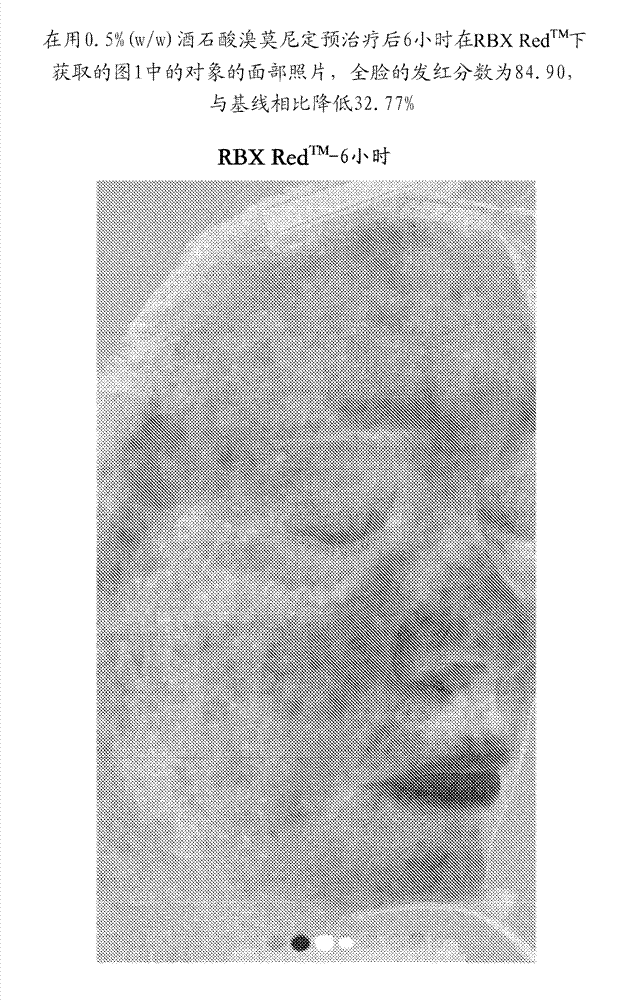
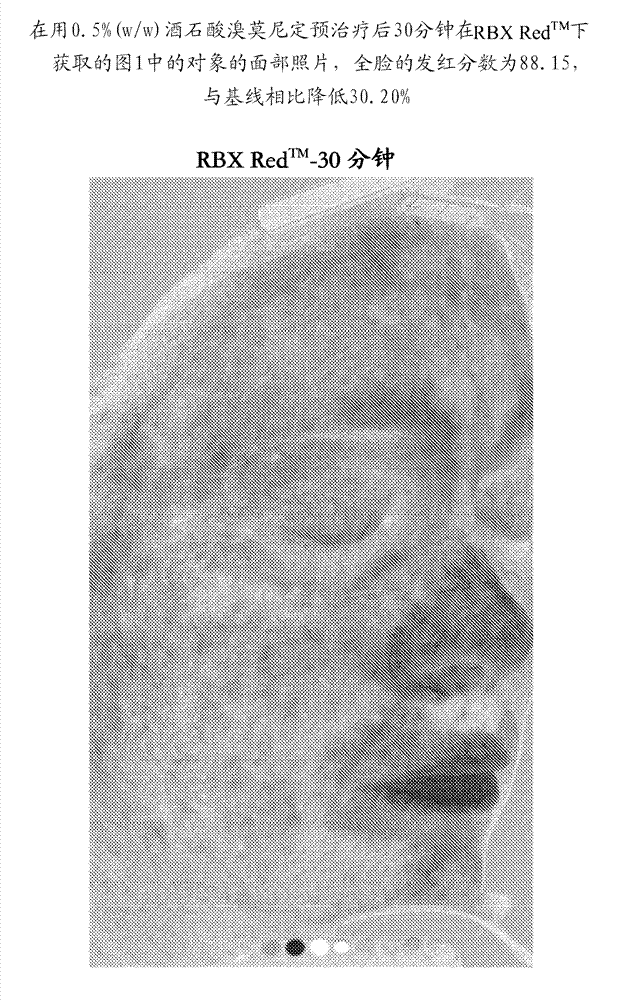
Improved methods and compositions for safe and effective treatment of telangiectasia
A kind of technology of brimonidine and brimonidine tartrate, applied in the field of compositions containing brimonidine for treating erythema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] gel topical formulations
[0093] This example illustrates gel topical formulations useful in the present invention.
[0094] A first set of gel formulations is described in Table 1 below.
[0095] Element % (w / w) % (w / w) % (w / w) Brimonidine tartrate 0.3 – 0.6% 0.6 – 3% 3 – 10% Methylparaben NF 0.15% 0.20% 0.10% Propylparaben NF 0.03% 0.02% 0.04% Hydroxyethyl Cellulose NF 1.0% 1.25% 1.5% Butanediol 1,3 3.0% 6.0% 18.0% glycerin 2.0% 4.0% 12.0% Disodium EDTA USP 0.05% 0.05% 0.05% Purified water, USP QS QS QS total 100% 100% 100%
[0096] The pH of the formulation is adjusted to approximately 4.5 to 7.0.
[0097] A second set of gel formulations is described in Table 2 below.
[0098] Element % (w / w) % (w / w) % (w / w) Brimonidine tartrate 0.3 – 0.6% 0.6 – 3.0% 3.0 – 10% Methylparaben 0.20% 0.20% 0.20% Propylparaben 0.05% 0....
Embodiment 2
[0107] Comparative bioavailability and pharmacokinetic studies of brimonidine compositions
[0108] This study administered brimonidine tartrate (ophthalmic solution (0.2%) and topical gel (0.07%, 0.18 % and 0.50%)) randomized, assessor-blinded, intrasubject comparative pharmacokinetic study. Main inclusion criteria included a clinical diagnosis of moderate to severe facial erythema associated with rosacea, a CEA score ≥3, and an IOP level of 11-21 mmHg. Intra-subject comparisons of topical and ocular exposure following 1 day of treatment with brimonidine tartrate ophthalmic solution 0.2% were performed.
[0109] A total of 102 subjects were randomly assigned: 24, 26, 25 and 27 subjects were treated with 0.5% Gel QD, 0.18% Gel BID, 0.18% Gel QD and 0.07% Gel BID, respectively. At the Day 1 visit, administer 1 drop of brimonidine tartrate ophthalmic solution 0.2% to each eye every 8 hours during a 24-hour period. After a 2-day washout period, 1 gram of topical gel (brimoni...
Embodiment 3
[0128] Clinical study on brimonidine tartrate gel composition
[0129] clinical research
[0130] A single-dose, randomized, double-blind, parallel-group, vehicle-controlled, dose-determining study was conducted to evaluate three brimonidine tartrate containing three concentrations (ie, 0.07%, 0.18% and 0.50% by weight) of three Pharmacodynamics and safety of a gel formulation in stable patients with moderate-to-severe erythematotelangiectatic rosacea.
[0131] A total of 122 subjects were randomized to receive one of three topical gel formulations (0.5%, 0.18%, 0.07% N=31, N=31, N=28) or vehicle gel (N=32) One-time application. Apply to the face in a single application in the morning. Subjects remained on site for a mandatory 12-hour post-dose observation period. All 122 subjects completed the study and were included in the intention-to-treat (ITT) and safety populations. A total of 117 subjects (96%) were included in the population point (PP) population.
[0132] E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com