Inhibition of cyp3a drug metabolism
A technique of drug and pharmacokinetics, applied in metabolic diseases, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as increasing the risk of drug-drug interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1: In vitro evaluation of boceprevir as an inhibitor of human cytochrome P450 enzymes
[0139] 1.1 Introduction and Purpose.
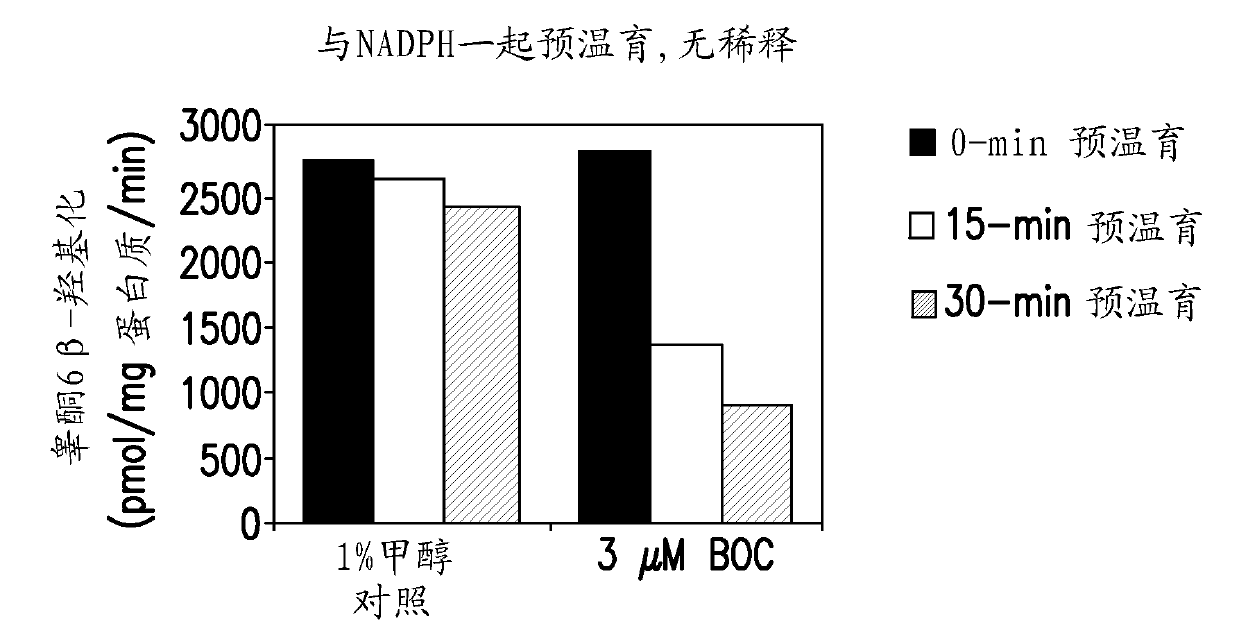
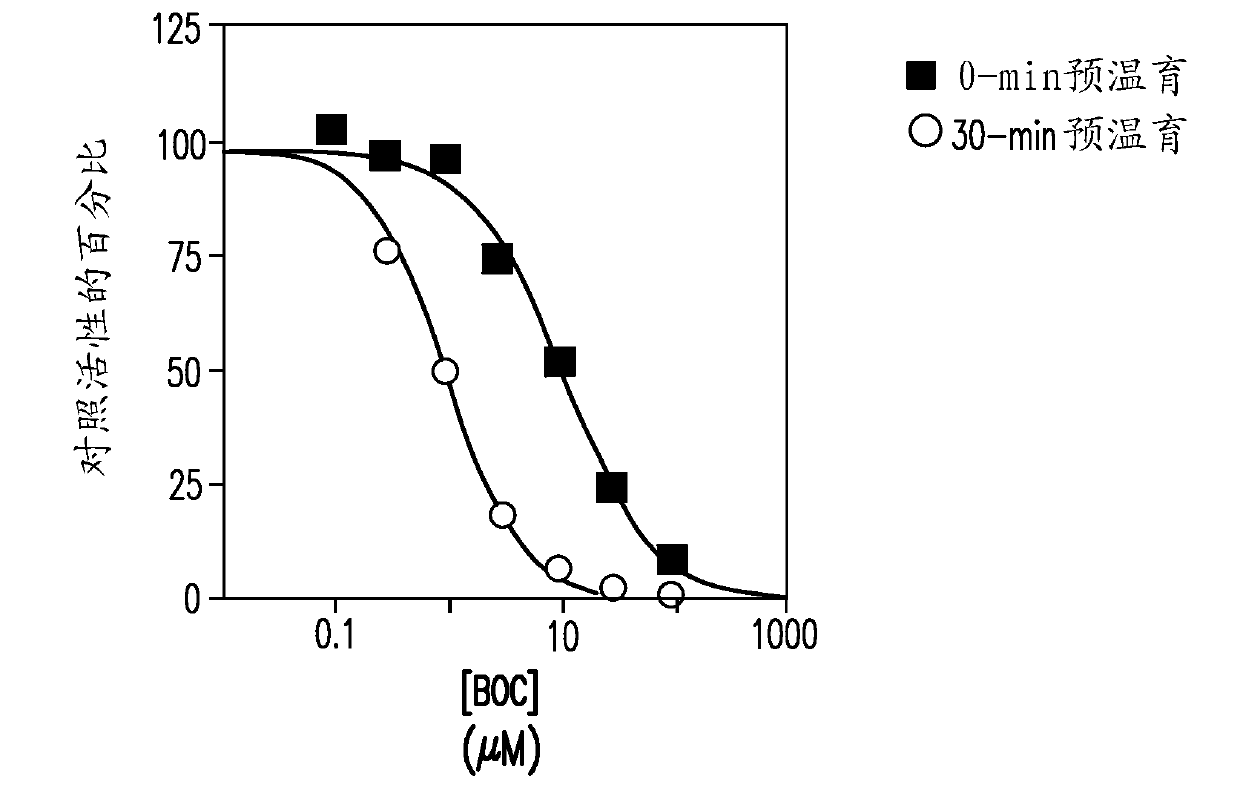
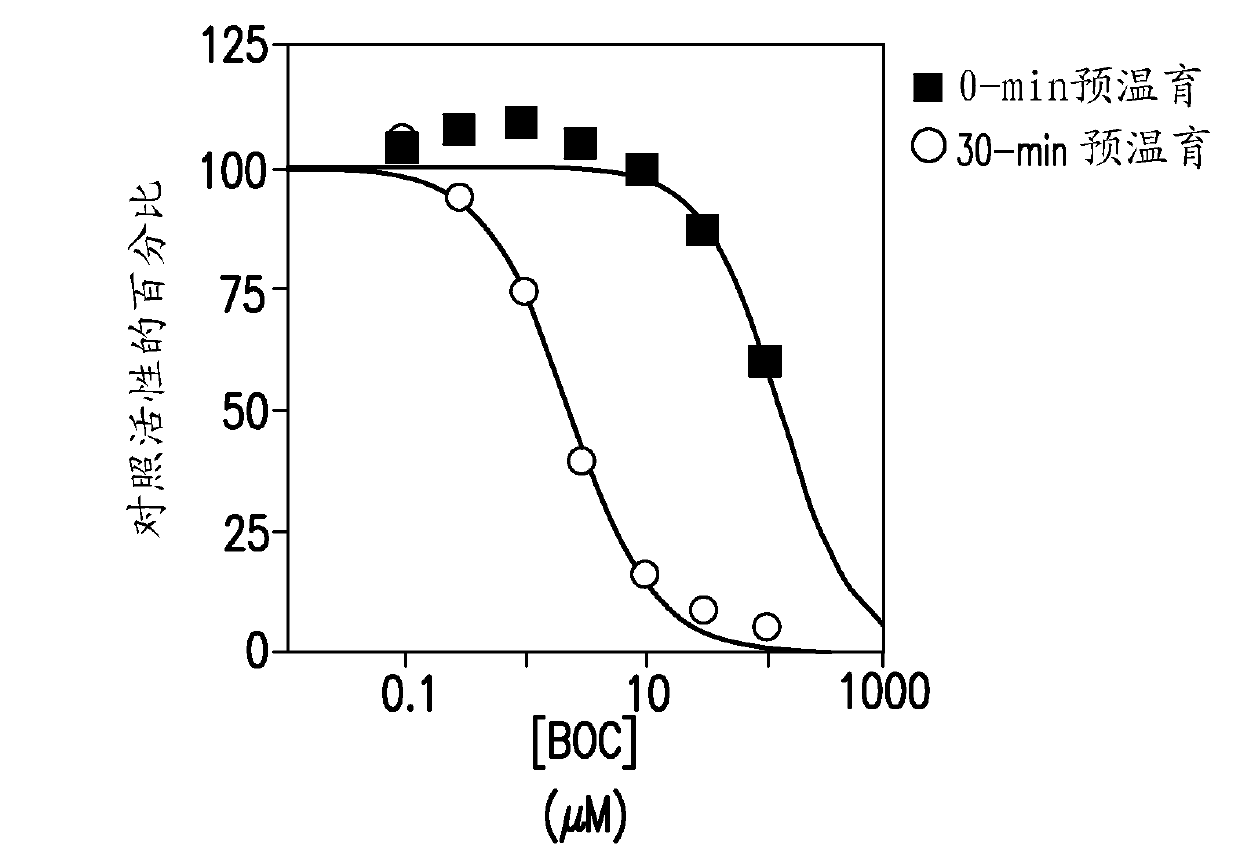
[0140] This study was designed to evaluate the ability of boceprevir to inhibit major CYP enzymes in human liver microsomes, with the aim of determining the potential of boceprevir to inhibit the metabolism of other drugs. The inhibitory capacity of boceprevir was determined in vitro by measuring the activity of each CYP enzyme in human liver microsomes in the presence or absence of boceprevir. These in vitro experiments were designed to measure the inhibition constant (IC) of boceprevir for direct inhibition of each human CYP enzyme examined. 50 value), and was designed to determine whether boceprevir is a time-dependent inhibitor of the enzyme. K for direct inhibition of CYP3A4 / 5 determined i Value and mechanism of inhibition (measured by midazolam 1'-hydroxylation). Experiments were also performed to determine whether the observed...
Embodiment 2
[0232] Example 2: Clinical evaluation of boceprevir (BOC) as an inhibitor of human cytochrome P450 enzymes
[0233] A clinical study was conducted to determine the effect of boceprevir on the pharmacokinetic (PK) profile of midazolam (MDZ) by monitoring the effect of boceprevir on MDZ, a sensitive CYP3A4 / 5 Substrate) metabolism to assess the ability of boceprevir to inhibit CYP3A4 / 5 in vivo.
[0234] 2.1 General methodology
[0235] The study was performed in healthy adult subjects (7 male and 5 female subjects) at a single center according to good clinical practice. This study used a fixed-sequence design (boceprevir alone followed by MDZ + boceprevir). Determine the PK profile of MDZ and its metabolite (1-hydroxymidazolam [1-OH-MDZ]) when administered alone and after coadministration with boceprevir and with boceprevir The PK characteristics after the 7-day washout period after Weiwei were compared.
[0236] 2.2 Test product, dose, administration mode
[0237] Administe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com