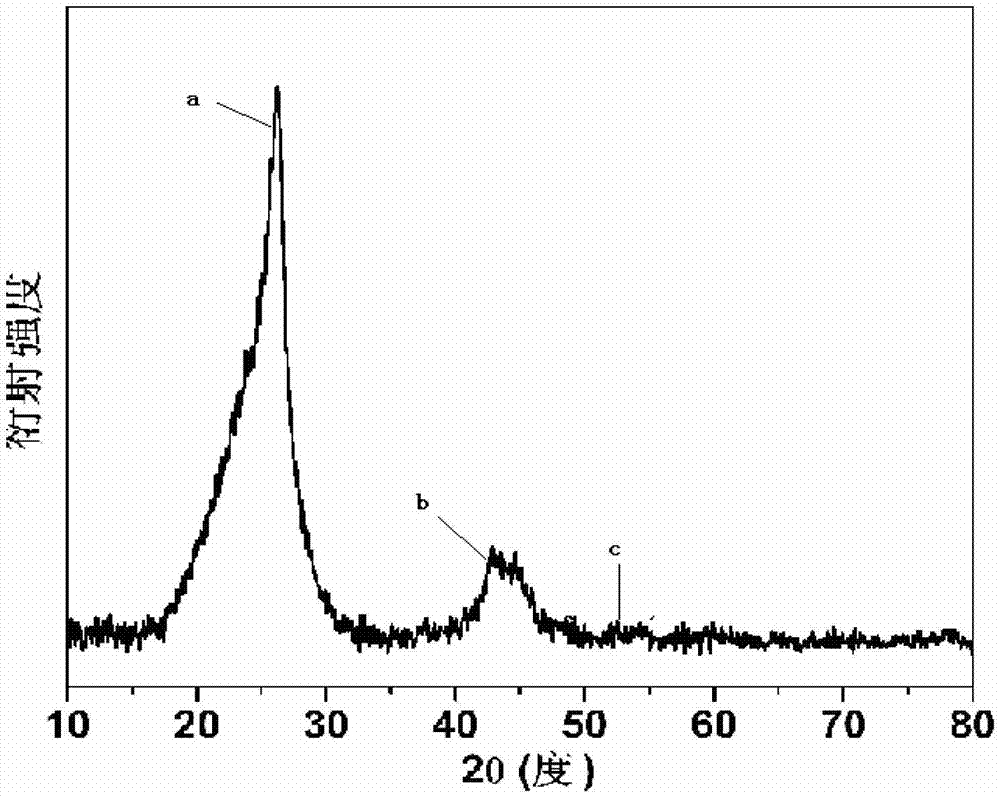
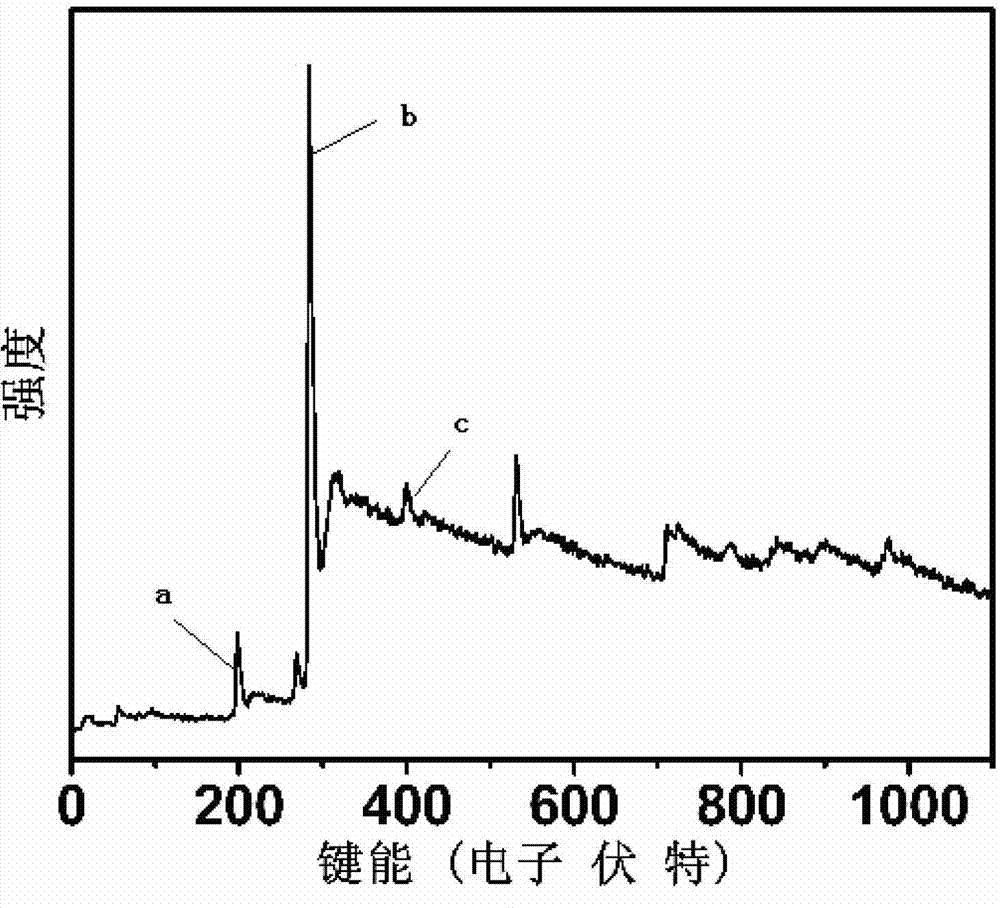
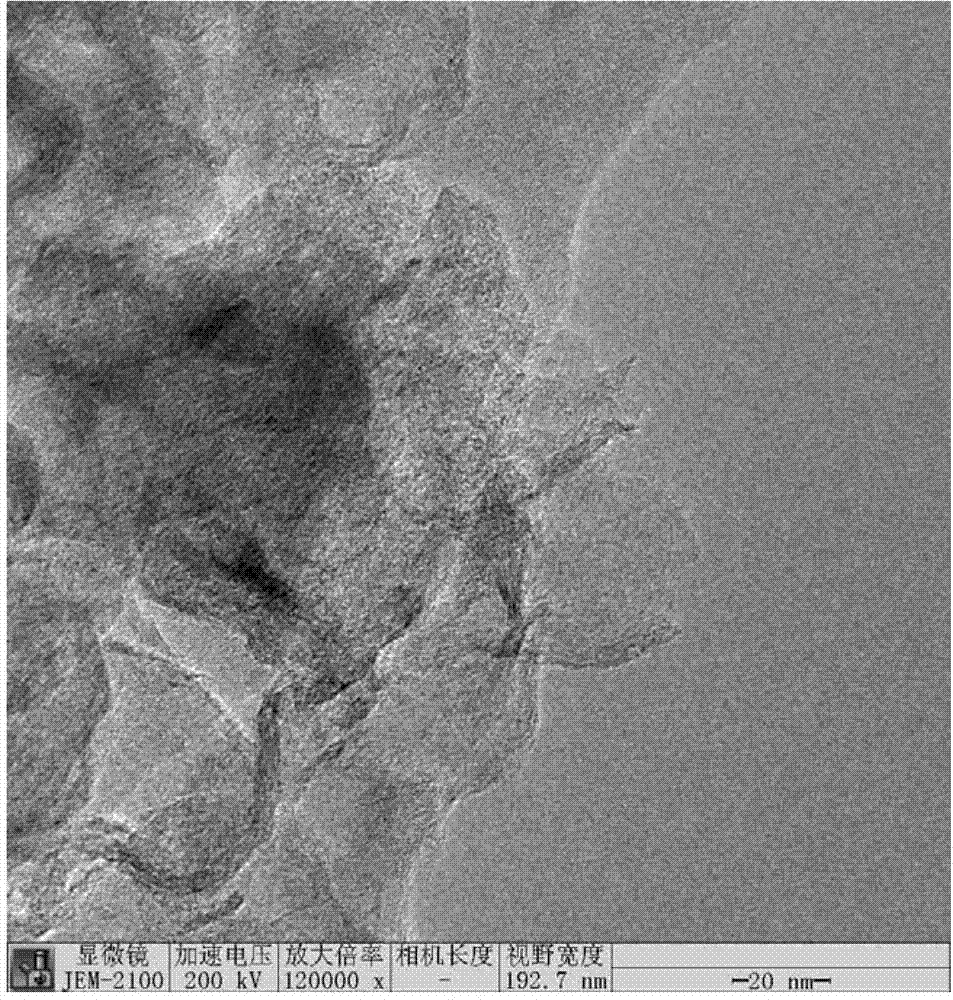
Method for synthesizing boron and nitrogen co-doped graphitized nano-carbon by using ion-exchange resin
An ion exchange resin, exchange resin technology, applied in nanocarbon, chemical instruments and methods, nanotechnology for materials and surface science, etc., can solve the problems of low yield, harsh reaction conditions, uncontrollable boron and nitrogen content, etc. , to achieve the effect of high output, low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0015] Specific embodiment one: the present embodiment utilizes the method for synthesizing boron-nitrogen co-doped graphitized nano-carbon of ion-exchange resin, is completed by the following steps: Carry out pretreatment, obtain the ion exchange resin after pretreatment;
[0016] 2. Add the pretreated ion exchange resin into the solvent, then add the boron-containing compound and graphitized catalyst at a temperature of 25-80°C and a stirring speed of 100-300r / min, and then stir for 6 ~30h, get the precursor;
[0017] 3. Pre-carbonize the precursor in step 2 for 1-6 hours at a temperature of 200-500°C under the protection of an inert gas to obtain a pre-carbonized precursor;
[0018] 4. Raise the temperature from room temperature to 550-1400°C at a rate of 2-15°C / min, and then heat-treat the pre-carbonized precursor at 550-1400°C for 20-3000 minutes to obtain the heat-treated precursor; the heat-treatment atmosphere It is one of nitrogen, argon, helium and ammonia or a mix...
specific Embodiment approach 2
[0021] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the ion exchange resin in step 1 is an anion and cation exchange resin, a macroporous ion exchange resin, or a chelating ion exchange resin. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0022] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that the anion and cation exchange resin is acrylic weakly basic anion exchange resin, styrene strong basic anion exchange resin, amphoteric ion exchange resin or acrylic acid Cation exchange resin. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com