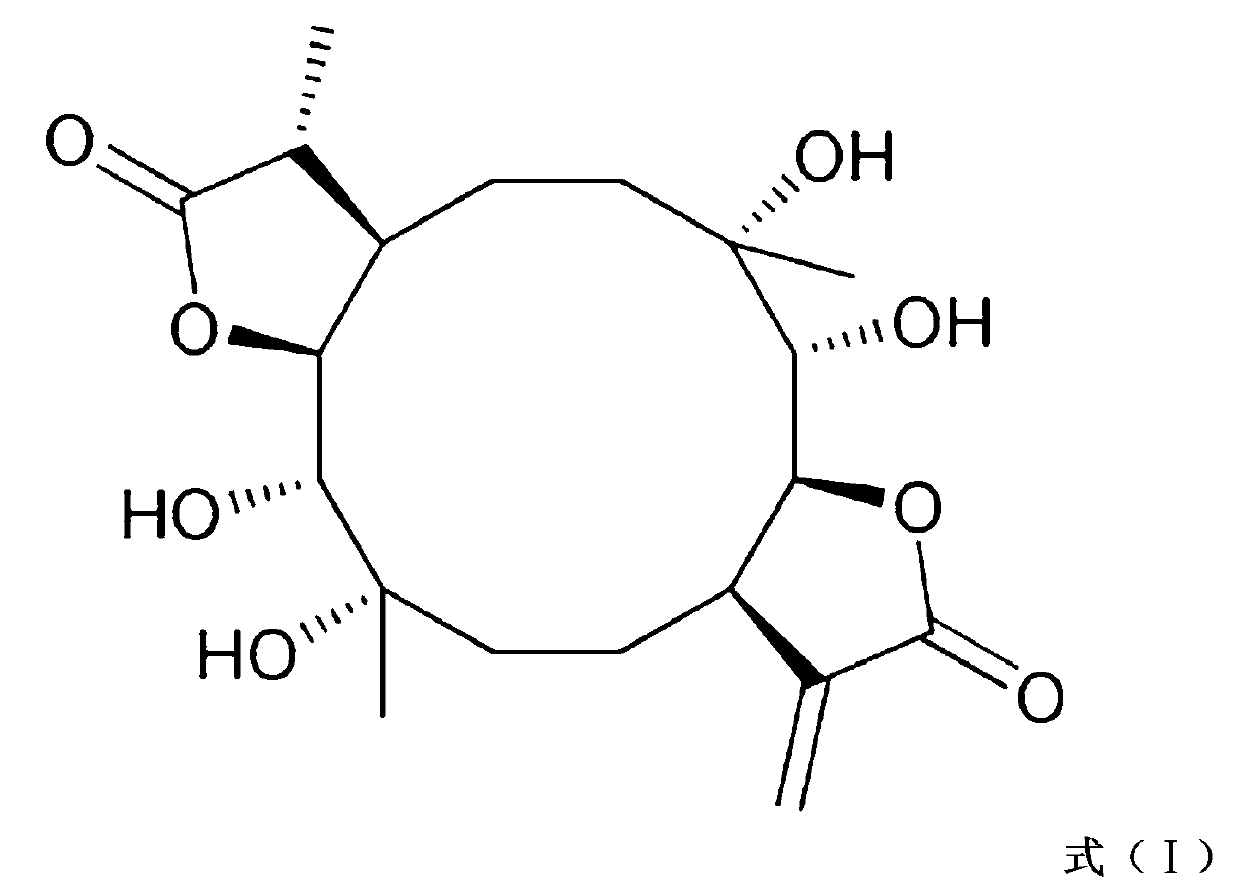
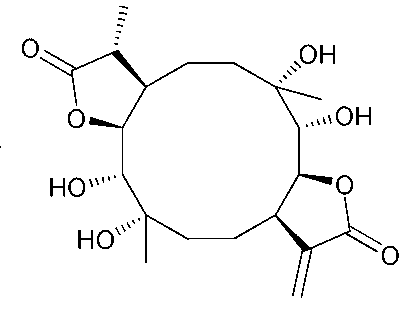
Application of Eryngiolide A in medicine for resisting tubercle bacillus
An anti-tuberculosis and drug technology, applied in the direction of antibacterial drugs, etc., to achieve the effects of strong tuberculosis inhibitory activity, expanded types, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Preparation of the compound Eryngiolide A tablet involved in the present invention:
[0017] Take 20 grams of compound Eryngiolide A, add 180 grams of conventional excipients for tablet preparation, mix well, and make 1000 tablets with a conventional tabletting machine.
Embodiment 2
[0018] Embodiment 2: the preparation of the compound Eryngiolide A capsule of the present invention:
[0019] Get 20 grams of compound Eryngiolide A, add conventional auxiliary materials for preparing capsules such as 180 grams of starch, mix well, and pack into capsules to make 1000 tablets.
[0020] The following pharmacodynamic experiments will further illustrate its drug activity.
[0021] The present invention will be further described below in conjunction with specific examples, but the specific examples do not limit the present invention in any form.
experiment example 1
[0022] Experimental Example 1 Determination of the Absolute Concentration of Eryngiolide A Against Bacillus Calmette-Guerin (BCG) by the Solid Medium Dilution Method
[0023] Scrape the BCG culture from the slant, add it to 3ml Middlebrook7H9 broth medium, add a small amount of glass beads, tighten the test tube cap, vibrate and grind vigorously on the vortex oscillator, and compare it with the standard McFarland turbidimetric tube (MacFarland No.1 ) turbidity, that is, to prepare 1mg / ml bacillus Calmette-Guerin (BCG) bacterial suspension.
[0024] Make Eryngiolide A into a high-concentration stock solution with DMSO, dilute the stock solution to the required concentration with 5% Tween-80 sterile ultrapure water, and add the diluted Eryngiolide A to 4ml Middlebrook7H11 agar medium according to the required dose (The medium has been sterilized by high pressure steam at 121°C for 15 minutes, cooled to 50~55°C), mixed evenly to make Eryngiolide A, the concentrations are 6.0 ug / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com