Method for synthesizing tyrosine kinase inhibitor PCI-32765
A PCI-32765, tyrosine kinase technology, applied in the field of medicinal chemistry, can solve the problems of dangerous reaction, difficult purification, many side reactions, etc., and achieve the effects of mild reaction conditions, environmental friendliness and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
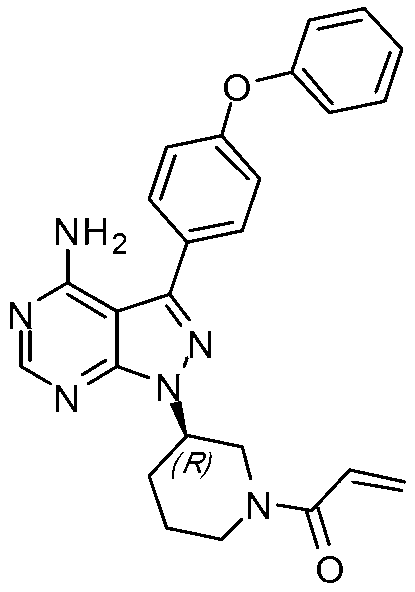
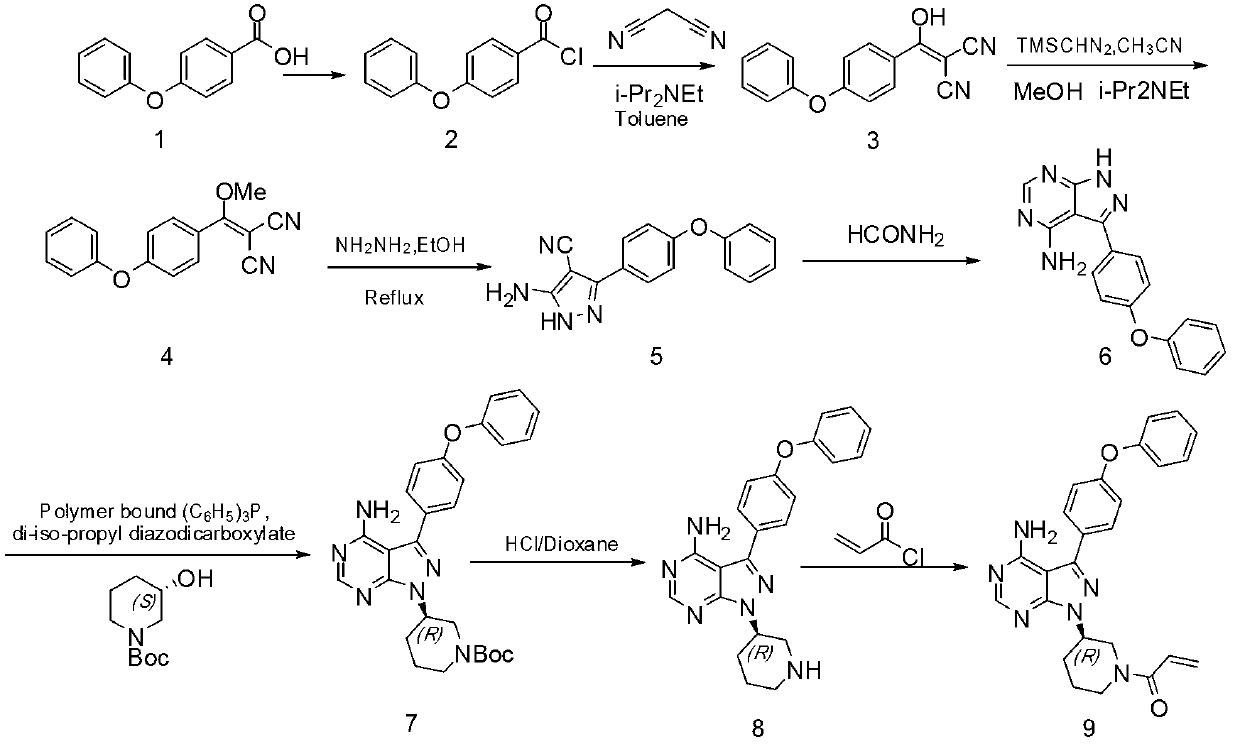
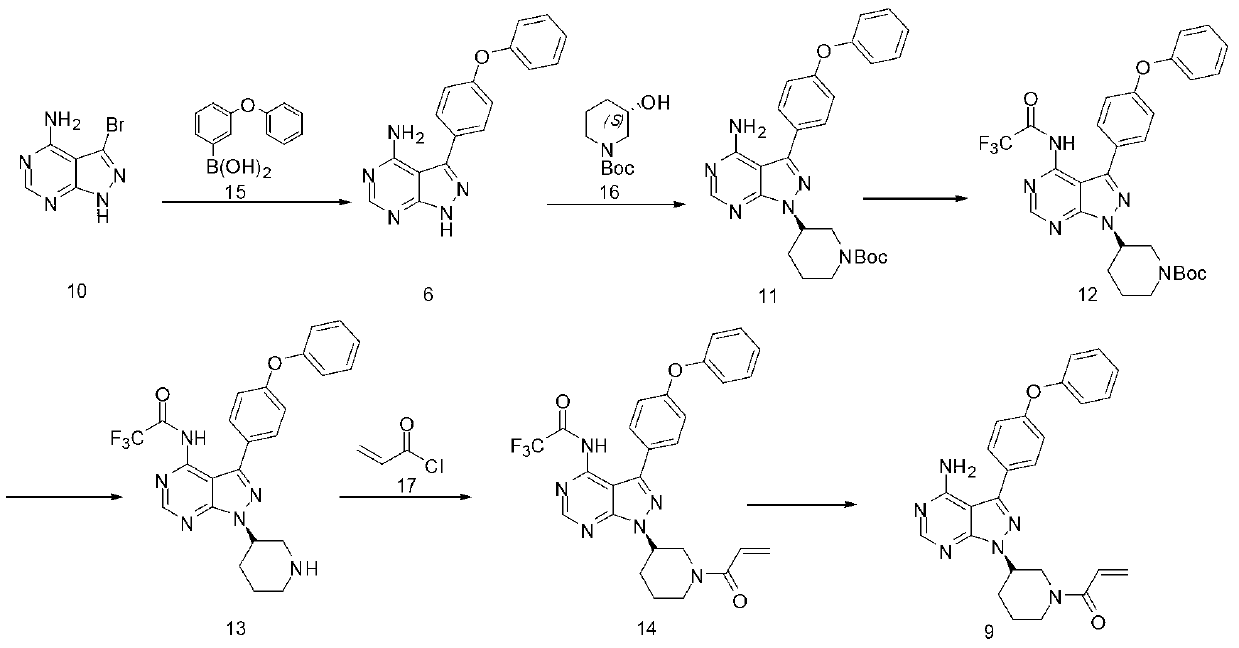
[0020] A synthetic method of tyrosine kinase inhibitor PCI-32765 comprises the following steps:
[0021] 1. Compound 10 and compound 15 undergo a coupling reaction to obtain compound 6;
[0022] 2. During the reaction of compound 6 and compound 16 to obtain compound 11, we chose a more ideal catalyst;
[0023] 3. Compound 11 was protected to obtain compound 12;
[0024] 4. Selective deprotection of compound 12 to obtain compound 13;
[0025] 5. Compound 13 has only the only position available for compound 17 to attack, and a very pure compound 14 is obtained;
[0026] 6. Take off the protecting group to get PCI-32765
[0027]
[0028] Among them, compounds 10, 15, 16, and 17 can be synthesized using reagents or industrial grade compounds, or using related methods and techniques.
[0029] In step 1) of the present invention, compound 10 and compound 15 undergo a Suzuki coupling reaction in a suitable solvent in the presence of an alkaline reagent and a catalyst to genera...
Embodiment 1
[0034] Preparation of compound 6
[0035] Under nitrogen protection, add 0.1 moL of compound 10, 1.5 equivalents of compound 15 and 800 mL of dioxane into a 2L reaction flask, then add 1.5 equivalents of sodium acetate and 0.2 equivalents of catalyst PdCl2(PPh3)2, and react at 50-60°C for 5 hour, filtered while hot, and the filter residue was washed three times with ethanol, and the combined filtrate was concentrated to obtain a solid, which was rinsed with ethanol to obtain 16.2 grams of pure product, yield 60%
Embodiment 2
[0037] Preparation of compound 6
[0038] Under nitrogen protection, add 0.1 moL of compound 10, 1.5 equivalents of compound 15 and 800 mL of DMF into a 2L reaction flask, then add 1.5 equivalents of sodium acetate and 0.2 equivalents of catalyst PdCl2(PhCN)2, and react at 50-60°C for 5 hours. Filtrate hot, wash the filter residue three times with ethanol, combine the filtrates, concentrate to obtain a solid, rinse with ethanol to obtain 21.5 g of pure product, and the yield is 71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com