Method for determining effectiveness of medicine containing antibody as component
A determination method and a technology for pharmaceuticals, applied in the field of tissue staining, can solve problems such as low effect, and achieve the effect of promoting cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
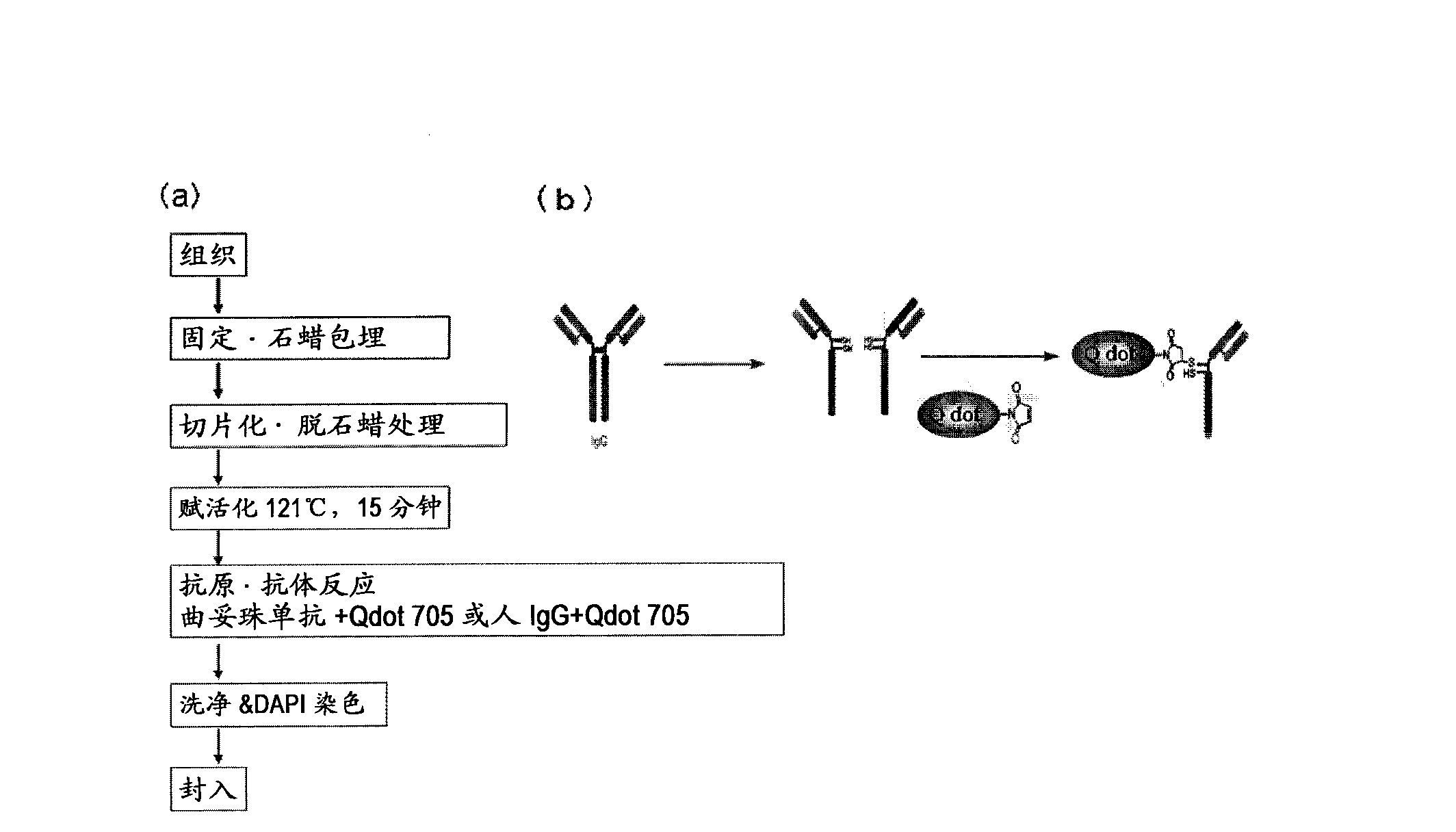
[0090] Method for preparing tissue sample immunostained using IHC-QDs method
[0091]As human breast cancer pathological tissues, 6 cases with a score of 0, 6 cases with a score of 1, 11 cases with a score of 2, and 14 cases with a score of 3 based on the IHC-DAB method were selected, totaling 37 cases. In addition, regarding the background of the case, as shown in Table 1, it is considered that there is no bias. With respect to the tissues of the above 37 cases, tissue samples were prepared according to the method used in usual histopathological diagnosis. That is, the mammary tissue test sample of a cancer patient is fixed in formalin, dehydrated with ethanol, then processed with xylene, immersed in high-temperature paraffin and embedded in paraffin, and the tissue sample ( figure 1 (a)). Next, the above-mentioned tissue samples were sectioned at 2 to 4 μm, deparaffinized with xylene, treated with ethanol, and washed with deionized water. In order for the antibody to effi...
Embodiment 2
[0097] Problems with fluorescent immunohistostaining
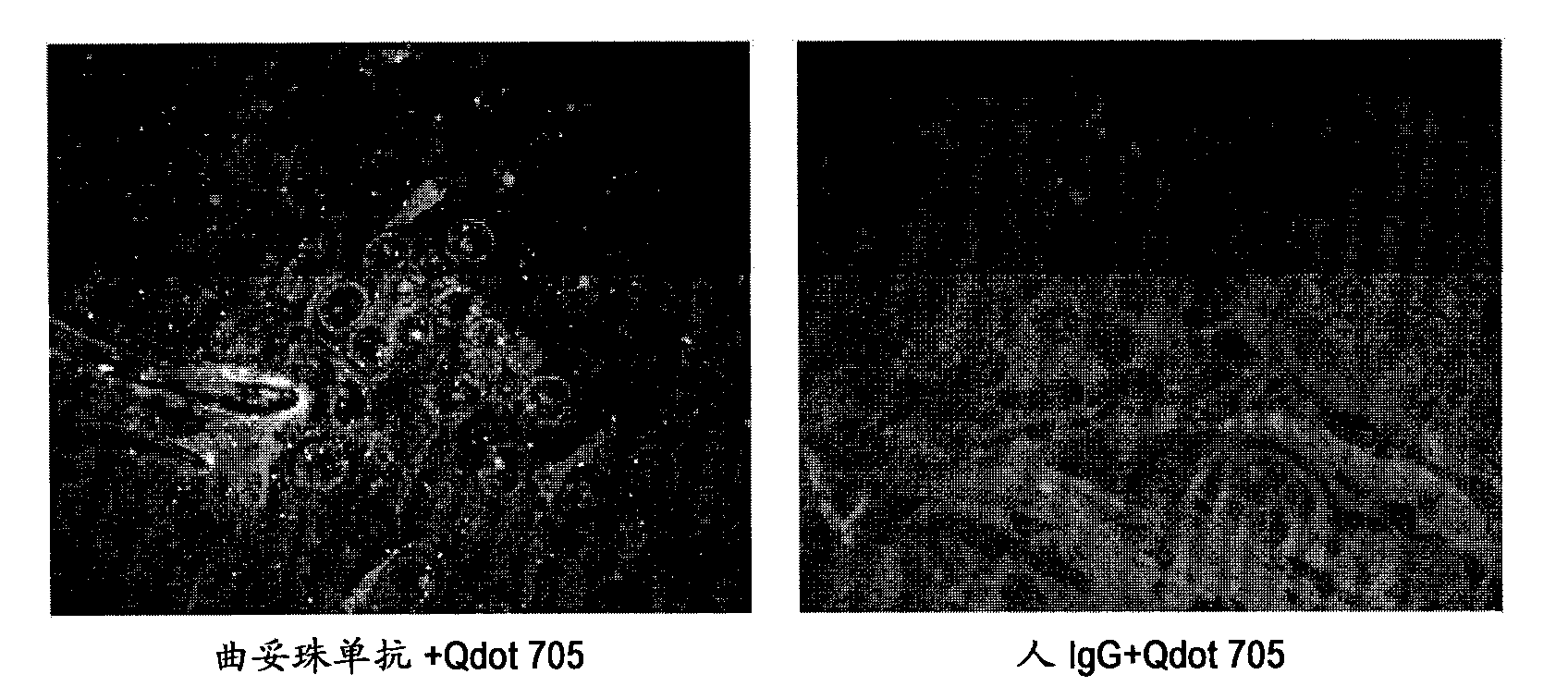
[0098] For the immunostained tissue sample prepared in Example 1 above, a combination of a confocal unit (manufactured by Yokogawa Electric Corporation), a fluorescence microscope (manufactured by Olympus Corporation), and an electron multiplier CCD (EM-CCD) imaging device (Andor (manufactured by the company) was irradiated with excitation light at 488nm, and then a fluorescence image (fluorescence still image) of quantum dot fluorescent particles (labeled to trastuzumab) was obtained using a bandpass filter with a wavelength acquisition range of 695 to 740nm ). figure 2 In the case of the case evaluated as score 3 by the IHC-DAB method as an example, it was shown that the fluorescence of quantum dot fluorescent particles was hardly observed when human IgG+Qdot705 was used as a control, but in contrast, trastuzumab+Qdot705 was used The fluorescence of quantum dot fluorescent particles was observed at Qdot705. These resu...
Embodiment 3
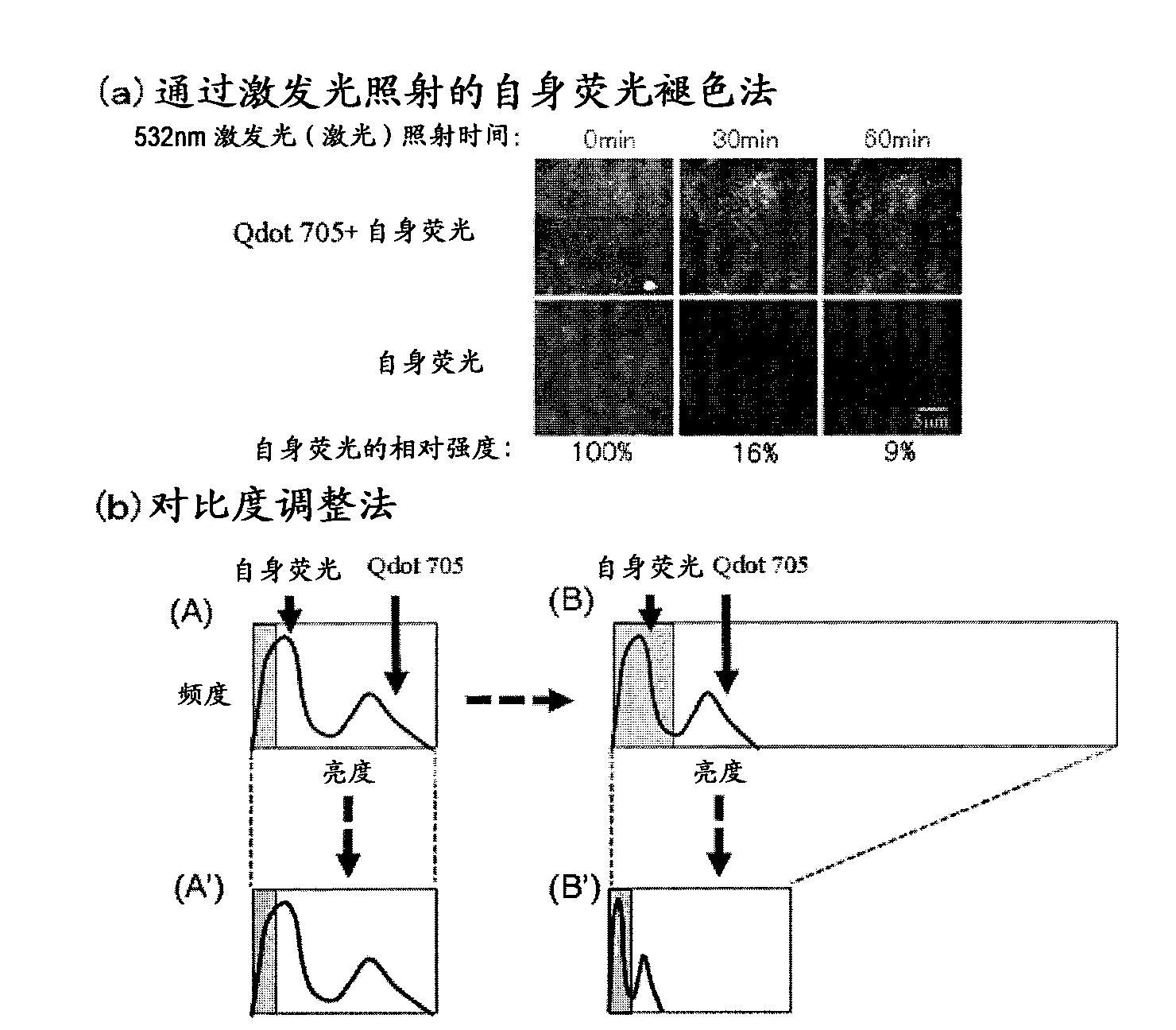
[0101] Creation of corrected fluorescence images with autofluorescence removed
[0102] Fluorescence still images with zero (0) fluorescence intensity of autofluorescence as background are required. In this way, the total fluorescence in the fluorescence still image can be calculated as the fluorescence derived from the quantum dot fluorescent particles. The contrast adjustment method of the above-mentioned embodiment 2 is a division method of fluorescence intensity, and zero (0) cannot be obtained by division, so it must be an image processing method using a subtraction method that can obtain zero (0). Thus, as an image processing method for removing autofluorescence in a fluorescent still image, the method described below is considered. First, the breast cancer tissue sample immunostained with quantum dot fluorescent particles is irradiated with excitation light (laser) with an excitation wavelength of 488 nm, and a fluorescence image of quantum dot fluorescent particles is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com