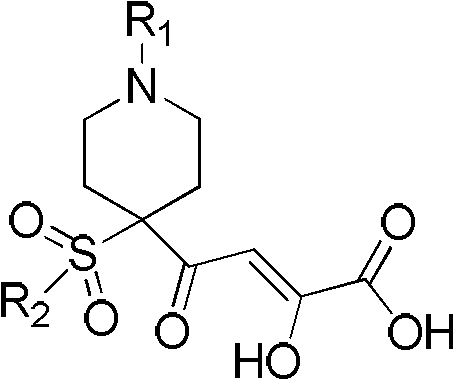
2-hydroxy-4-oxo-2-butenoic acid compound and pharmaceutical application thereof
A technology of compound and hydroxyl, which is applied in the field of preparation of intermediates or prodrugs of compounds, anti-influenza pharmaceutical applications, and can solve problems such as unclear mechanism of action, complex and changeable traditional Chinese medicine ingredients, and poor reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
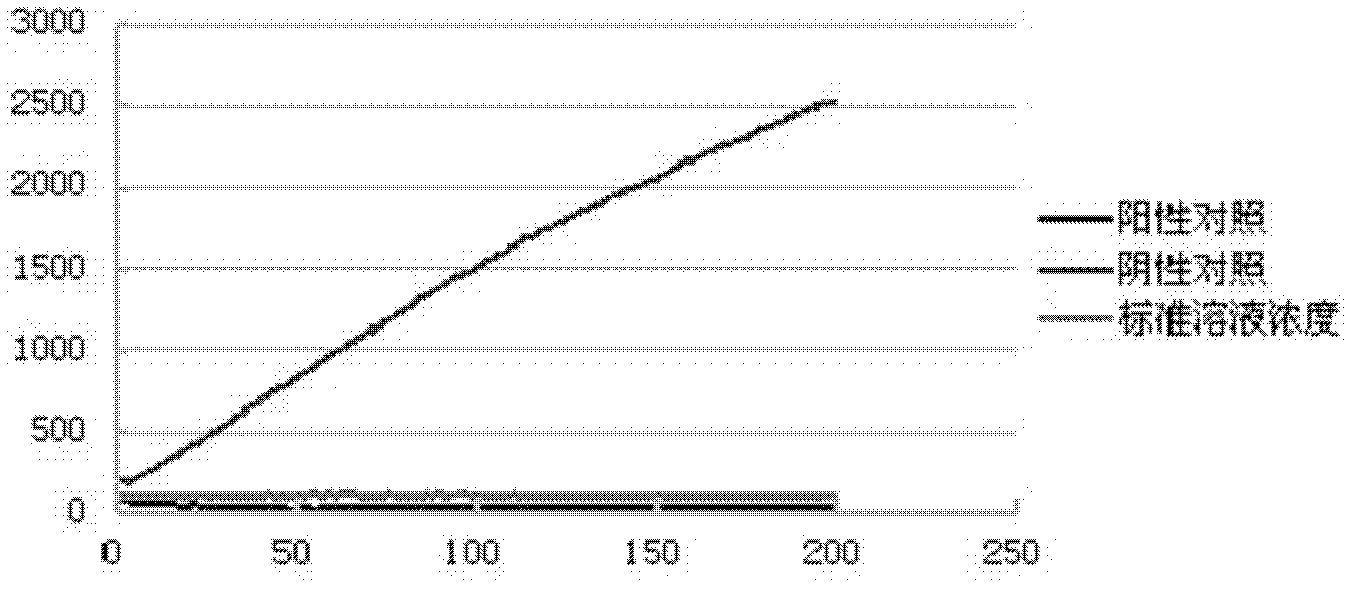
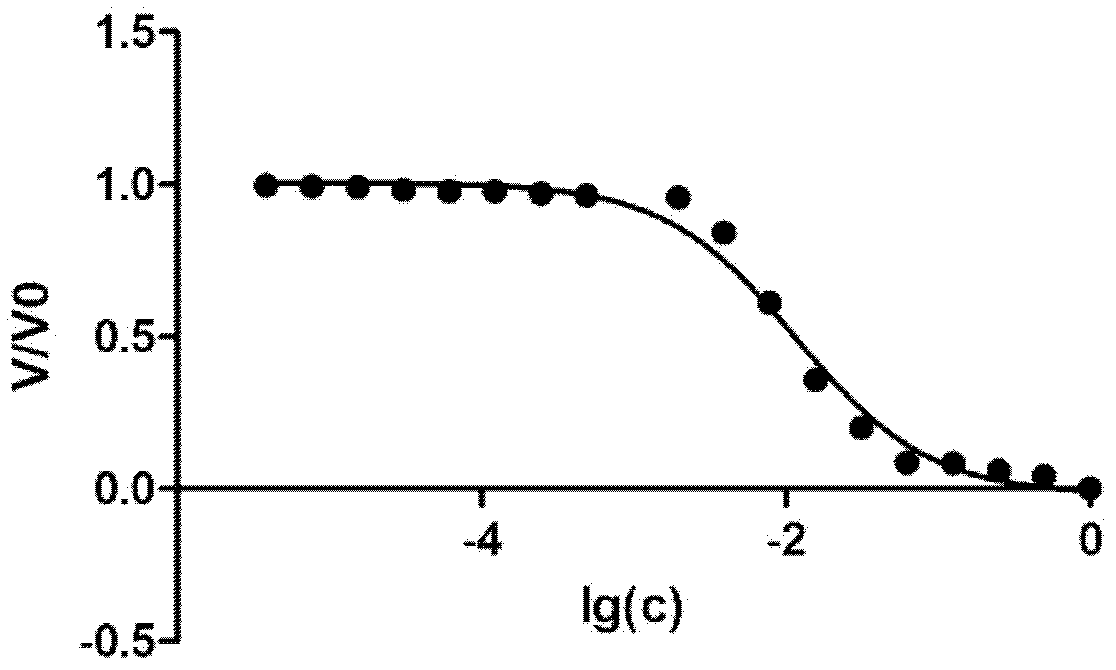
Image
Examples
Embodiment 1
[0064] Preparation of compound 4-[(1-tert-butoxycarbonyl-4-benzenesulfonyl)-piperidin-4-yl]-2-hydroxy-4-oxo-2-butenoic acid
[0065] A. Sodium chloride (4.2g, 0.105mmol) was dissolved in 10ml chloroform and 12.5ml water, and pipecolic acid ethyl ester (2.48g, 15.8mmol) and di-tert-butyl dicarbonate (3.63g, 16.6mmol), heated to reflux and stirred for 2.5h, cooled to room temperature, separated the organic phase, extracted the aqueous phase with chloroform (2×35ml), combined the organic phases, dried overnight with anhydrous sodium sulfate, concentrated under reduced pressure to obtain a light yellow oily liquid 1 .
[0066] B. Preparation of fresh LDA. Add a certain amount of dry diisopropylamine solvent, cool to -78°C, add an equal amount of n-butyllithium, continue stirring at this temperature for 0.5h, and add a certain amount of dry tetrahydrofuran during the reaction. Fresh LDA (2.31mmol) was dissolved in 10ml of dry tetrahydrofuran, cooled to -78°C, and 1-tert-butyloxyc...
Embodiment 2
[0084]Preparation of compound 4-[(1-benzyl-4-benzenesulfonyl)-piperidin-4-yl]-2-hydroxy-4-oxo-2-butenoic acid
[0085] A. Sodium chloride (4.2g, 0.105mmol) was dissolved in 10ml chloroform and 12.5ml water, and pipecolic acid ethyl ester (2.48g, 15.8mmol) and di-tert-butyl dicarbonate (3.63g, 16.6mmol), heated to reflux and stirred for 2.5h, cooled to room temperature, separated the organic phase, extracted the aqueous phase with chloroform (2×35ml), combined the organic phases, dried overnight with anhydrous sodium sulfate, concentrated under reduced pressure to obtain a light yellow oily liquid 1 .
[0086] B. Preparation of fresh LDA. Add a certain amount of dry diisopropylamine solvent, cool to -78°C, add an equal amount of n-butyllithium, continue stirring at this temperature for 0.5h, and add a certain amount of dry tetrahydrofuran during the reaction. Fresh LDA (2.31mmol) was dissolved in 10ml of dry tetrahydrofuran, cooled to -78°C, and 1-tert-butyloxycarbonyl pipeco...
Embodiment 3
[0105] Preparation of compound 4-[(1-tert-butoxycarbonyl-4-p-fluorobenzenesulfonyl)-piperidin-4-yl]-2-hydroxy-4-oxo-2-butenoic acid
[0106] A. Sodium chloride (4.2g, 0.105mmol) was dissolved in 10ml chloroform and 12.5ml water, and pipecolic acid ethyl ester (2.48g, 15.8mmol) and di-tert-butyl dicarbonate (3.63g, 16.6mmol), heated to reflux and stirred for 2.5h, cooled to room temperature, separated the organic phase, extracted the aqueous phase with chloroform (2×35ml), combined the organic phases, dried overnight with anhydrous sodium sulfate, concentrated under reduced pressure to obtain a light yellow oily liquid 1 .
[0107]B. Preparation of fresh LDA. Add a certain amount of dry diisopropylamine solvent, cool to -78°C, add an equal amount of n-butyllithium, continue stirring at this temperature for 0.5h, and add a certain amount of dry tetrahydrofuran during the reaction. Fresh LDA (2.31mmol) was dissolved in 10ml of dry tetrahydrofuran, cooled to -78°C, and 1-tert-bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com