Purpose of ribosomal protein S5 in catalyzing of protein molecules for generatation of dephosphorylation reaction
A ribosomal protein and dephosphorylation technology, which is applied to peptide/protein components, medical preparations containing active ingredients, genetic material components, etc., can solve problems such as unreported effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
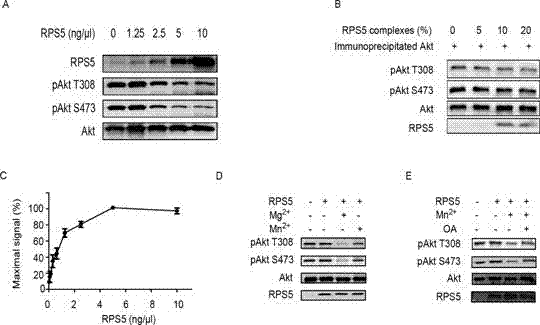
[0090] Example 1 RPS5 is the protein phosphatase of Akt
[0091] Take recombinant purified phosphorylated Akt (Millipore Company) and recombinant GST-RPS5 (Abnova Biologicals Company) or self-made purified recombinant His-RPS5 in 50mM Tris-HCl (pH 7.0), 10mM MgCl 2 Incubate for 30 minutes at 30oC in the buffer. The levels of phosphorylated Akt Thr308 and Ser473 were detected by Western blot. The results showed that RPS5 concentration-dependently dephosphorylated phosphorylated Akt ( figure 1 A), showing that RPS5 is an Akt protein phosphatase.
[0092] After hepatic stellate cell HSC-T6 was stimulated with TGFb1 (2ng / ml) for 4 hours, RPS5 and Akt were immunoprecipitated with RPS5 and Akt antibodies, respectively, and then different amounts of RPS5 immunoprecipitates were co-incubated with Akt precipitates, according to the above method Detection of RPS5 protein phosphatase activity. The results showed that endogenous RPS5 also catalyzed the dephosphorylation reaction of...
Embodiment 2
[0093] Example 2 Detection of RPS5 protein phosphatase activity with phosphorylated bisamide rhodamine-110 peptide
[0094] Use phosphorylated bisamide rhodamine-110 peptide in the Ser / Thr Phosphatase assay kit (Promega, Madison) as the substrate, and the dephosphorylation reaction buffer contains 2mM MnCl 2 , detect the phosphatase activity of RPS5 according to the reaction conditions of the kit. The fluorescence intensity was measured with a Synergy 2 multifunctional microplate reader (Biotek), and the enzyme activity was expressed as % of the maximum signal. The results showed that RPS5 concentration-dependently dephosphorylated phosphorylated bisamide rhodamine-110 peptide ( figure 1 C).
Embodiment 3
[0095] Example 3 RPS5 protein phosphatase activity depends on manganese and magnesium ions, and is insensitive to broad-spectrum protein phosphatase inhibitor okadaic acid
[0096] Take recombinant purified phosphorylated Akt (Millipore Company) or self-made purified recombinant His-RPS5 in 50mM Tris-HCl (pH 7.0) solution and incubate with okadaic acid (200 nM) for 10 minutes at 4oC. The RPS5 phosphatase activity was detected according to the conditions of Example 1. The results showed that RPS5 activity was dependent on divalent metal ions Mn and Mg ( figure 1 D), but the inhibitory effect of okadaic acid on its activity is weak ( figure 1 E), These properties of RPS5 are the same as those of PP2C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com