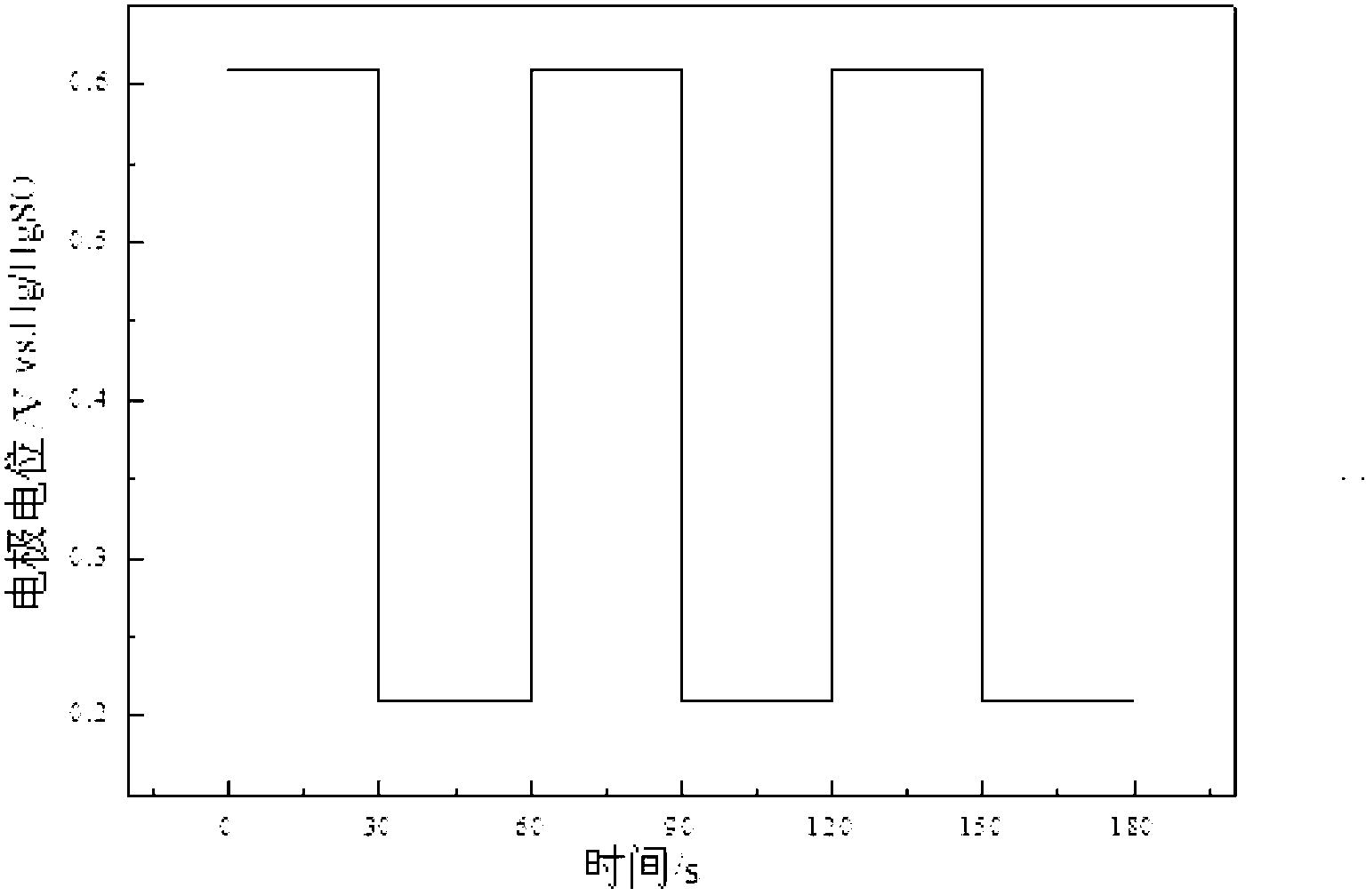
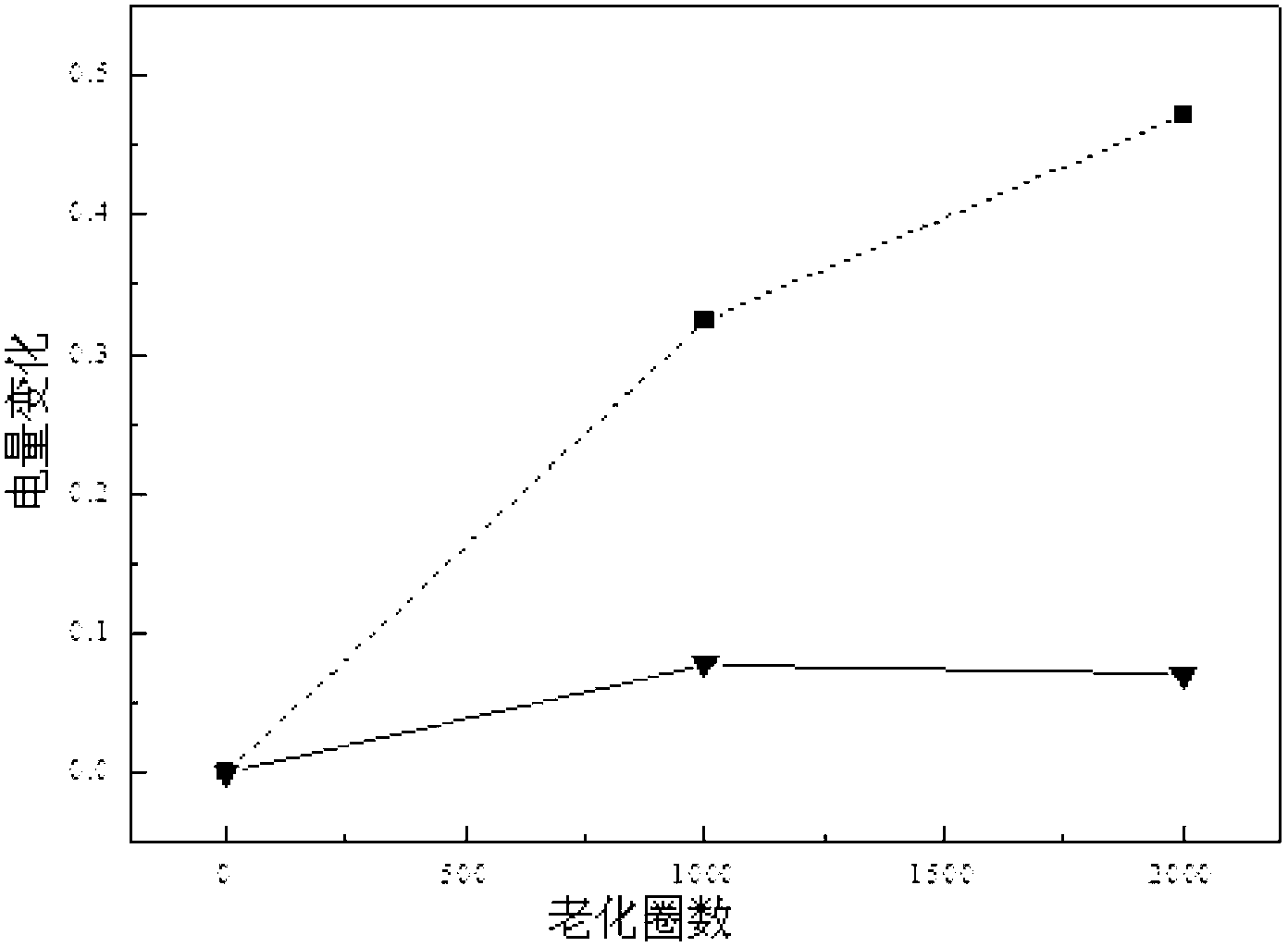
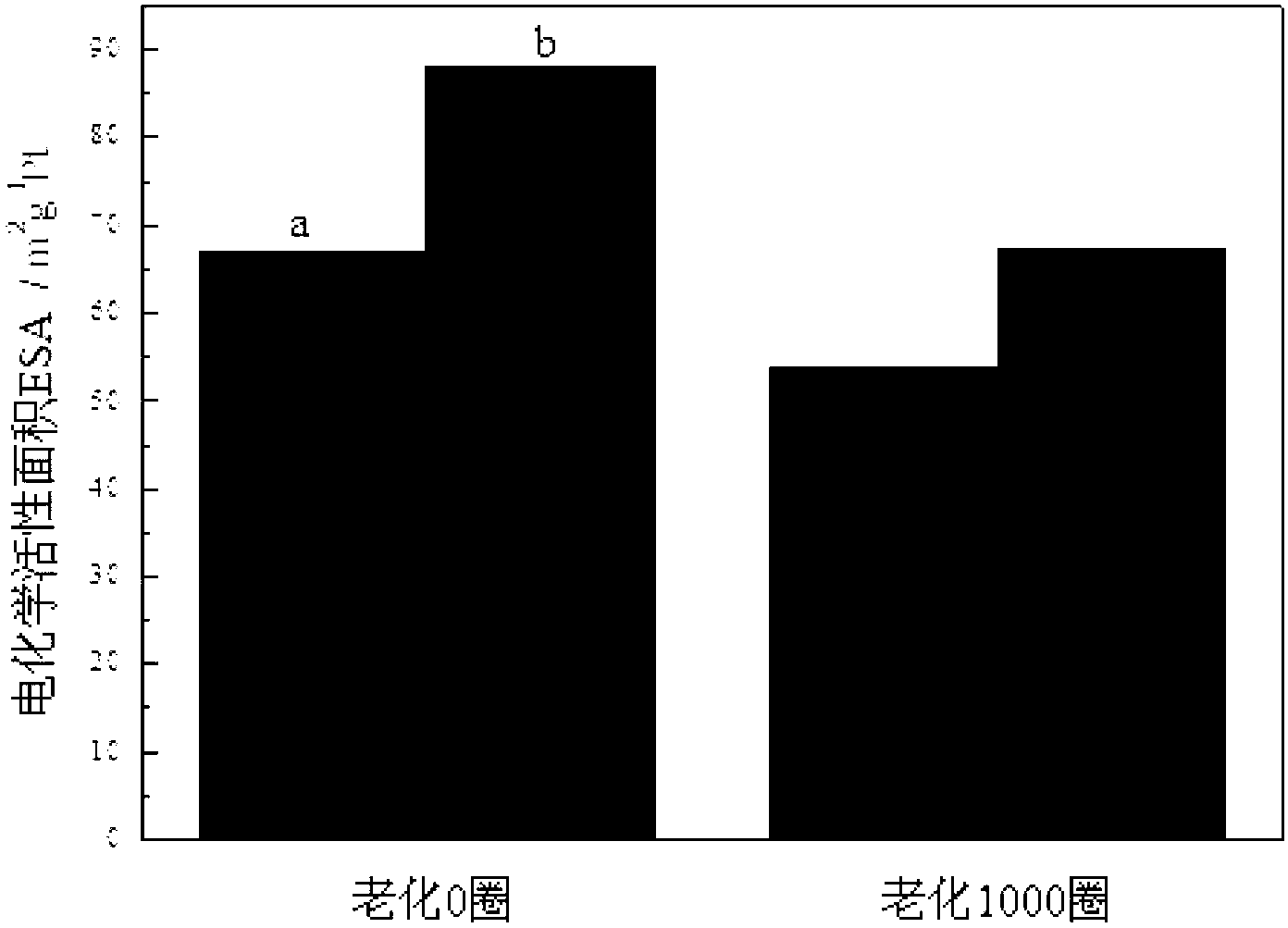
Preparation method of high-stability and high-activity carbon-supported Pt-based catalyst for fuel cell
A high stability, fuel cell technology, applied in the field of electrochemistry, can solve the problems of low catalyst stability, poor cycle stability, and difficult preparation process, etc., and achieve the effect of increased mass activity, good stability, and high cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0016] Specific embodiment one: the high stability of a kind of fuel cell of the present embodiment, the preparation method of highly active carbon-supported Pt-based catalyst is carried out according to the following steps:
[0017] 1. Pretreatment of the carbon carrier: cleaning the surface of the carbon carrier or oxidizing the carbon carrier to obtain the treated carbon carrier;
[0018] Two, the introduction of the carbon-nitrogen heterocyclic structure on the carbon carrier: the mass ratio of the carbon carrier after the treatment in step 1 and the carbon-nitrogen heterocyclic structure compound is 1: (1~100), and the carbon nitrogen heterocyclic structure compound after the treatment in step 1 The ratio of the mass of the carrier to the volume of the deionized water is 1g: (80-100) mL, and the volume ratio of the organic solvent to the deionized water is 1:1. After the heterocyclic compound is mixed, add deionized water and an organic solvent, and treat it for 0.5h to 1...
specific Embodiment approach 2
[0021] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the cleaning and removal of impurities on the surface of the carbon carrier described in step one is carried out as follows: Treat for 5h to 7h, and then vacuum dry to complete the pretreatment of the carbon carrier to obtain the treated carbon carrier. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0022] Specific embodiment three: what this embodiment is different from specific embodiment one or two is: the method described in step 1 is carried out oxidation treatment to carbon support is as follows: by the ratio of the mass of carbon support and the volume of concentrated nitric acid is 1g: (40-60) mL, add concentrated nitric acid to the carbon carrier, under the condition of 80-100 ° C, reflux for 1h-3h, then cool to room temperature, and then the ratio of the mass of the carbon carrier to the volume of deionized water is 1g: (100~500)mL, add deionized water to dilute, at room temperature, stir at a stirring speed of 100r / min~500r / min, stir for 10h~14h, and finally wash with deionized water until neutral, at a temperature of 70~ Under the vacuum condition of 90° C., dry for 9-11 hours to complete the pretreatment of the carbon carrier to obtain the treated carbon carrier. Other steps and parameters are the same as those in Embodiment 1 or 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistivity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com