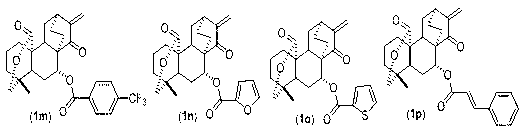
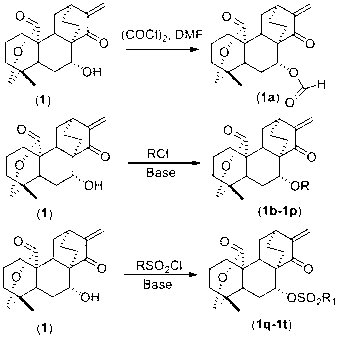
7-acyl-15-oxo-spiraea lactone derivative as well as preparation method and applications thereof
An acetyl and formyl technology, which is applied in the application field of preparing anti-tumor drugs, can solve the problems of strong drug resistance of tumor cells, drug resistance of cancer cells, and difficulty in effectively inhibiting the development or metastasis of cancer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a: in N 2 Oxalyl chloride ((COCl) 2 , 2.0eq, 28ul) into 10ml of dry dichloromethane (DCM), stirred at 0°C, slowly added N, N-dimethylformamide (DMF, 2.0eq, 23ul) dropwise, after the addition was completed, in After stirring at 0°C for 30 min, inject 50 mg of compound 1 (dissolved in 5 ml of dichloromethane solution) into the reaction system, move it to room temperature and stir for 2 hours, track it with TLC, and find that the raw material disappears, add 20 ml of water to quench Reaction, separate the dichloromethane layer, continue to extract the water layer with 20ml of dichloromethane once, combine the dichloromethane layers, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate, and obtain compound 1a through simple silica gel column chromatography (50.9 mg, 94% yield).
[0032] b: compound 1 (50mg), 4-dimethylaminopyridine (DMAP, 0.5eq, 9.2mg) was placed in a dry reaction bottle, and the sample was dissolved with 10ml of dry dichloromethane ...
Embodiment 2
[0035] Compound spectral data:
[0036] compound ( 1a ): 7-formylacyl-15-oxospirandrolactone, 1 H-NMR (400MHz, CDCl 3 ) δ: 8.05(1H, s), 5.95(1H, d, J=1.4Hz), 5.43(1H, dd, J=4.4, 10.8Hz), 5.25(1H, d, J=1.4Hz), 4.24( 1H, dd, J=2.4, 11.6Hz), 4.12(1H, d, J=11.6Hz), 3.14(1H, m), 2.81(1H, brs), 2.16-2.27(4H, m), 1.85-1.92 (1H, m), 1.60-1.78(5H, m), 1.53-1.57(1H, m), 1.33-1.48(3H, m), 1.18-1.26(1H, m), 0.95(3H, s). 13 C-NMR (100MHz, CDCl 3 ) δ: 200.6, 173.7, 160.4, 147.3, 117.3, 76.4, 70.6, 48.7, 46.4, 45.7, 44.6, 40.6, 37.3, 35.6, 32.8, 26.9, 25.8, 23.7, 23.4, 27 m / z 358.1781 [M] + (C 21 h 26 o 5 , calcd 358.1780).
[0037] compound ( 1b ): 7-acetylacyl-15-oxospirandrolactone, 1 H-NMR (400MHz, CDCl 3 ) δ: 5.84(1H, d, J=1.6Hz), 5.22(1H, dd, J=4.4, 11.6Hz), 5.15(1H, d, J=1.6Hz), 4.15(1H, dd, J=3.6 , 11.6Hz), 4.13(1H, d, J=11.6Hz), 3.05(1H, m), 2.72(1H, t, J=2.8, 2.8Hz), 2.05-2.16(3H, m), 1.92(3H , s), 1.68-1.82(1H, m), 1.08-1.68(11H, m), 0.86(3H, s). 13 C-NMR (100...
Embodiment 3
[0057] Inhibitory effects of compounds on various tumor cell lines:
[0058] (1) Experimental method
[0059] 1. Inoculate cells: Use culture medium (DMEM or RMPI1640) containing 10% fetal bovine serum to prepare a single cell suspension, inoculate 5000-10000 cells per well into a 96-well plate with a volume of 100 μl per well, and adherent cells in advance 12 hour inoculation.
[0060] 2. Add the compound solution to be tested (fixed concentration of 40 μM for primary screening, at which concentration the compound that inhibits the growth of tumor cells near 50% is set to enter the gradient rescreening at 5 concentrations), the final volume of each well is 200 μl, and each treatment is set at 3 multiple holes.
[0061] 3. Color development: After incubating at 37 degrees Celsius for 48 hours, add 20 μl of MTT solution to each well. Continue to incubate for 4 hours, terminate the culture, discard the culture supernatant in the well, add 200 μl of SDS solution (10%) to e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com