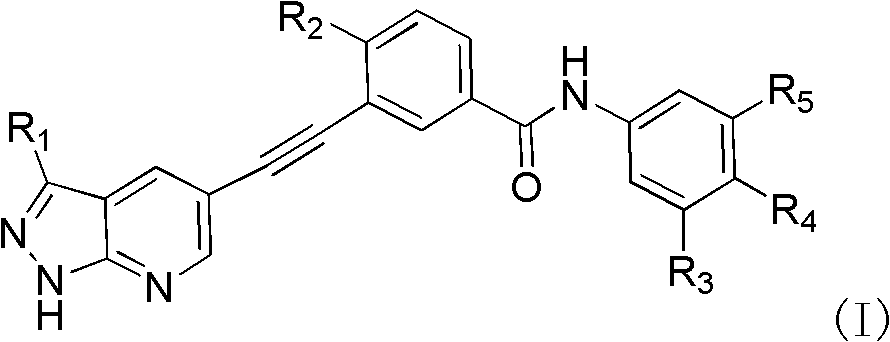
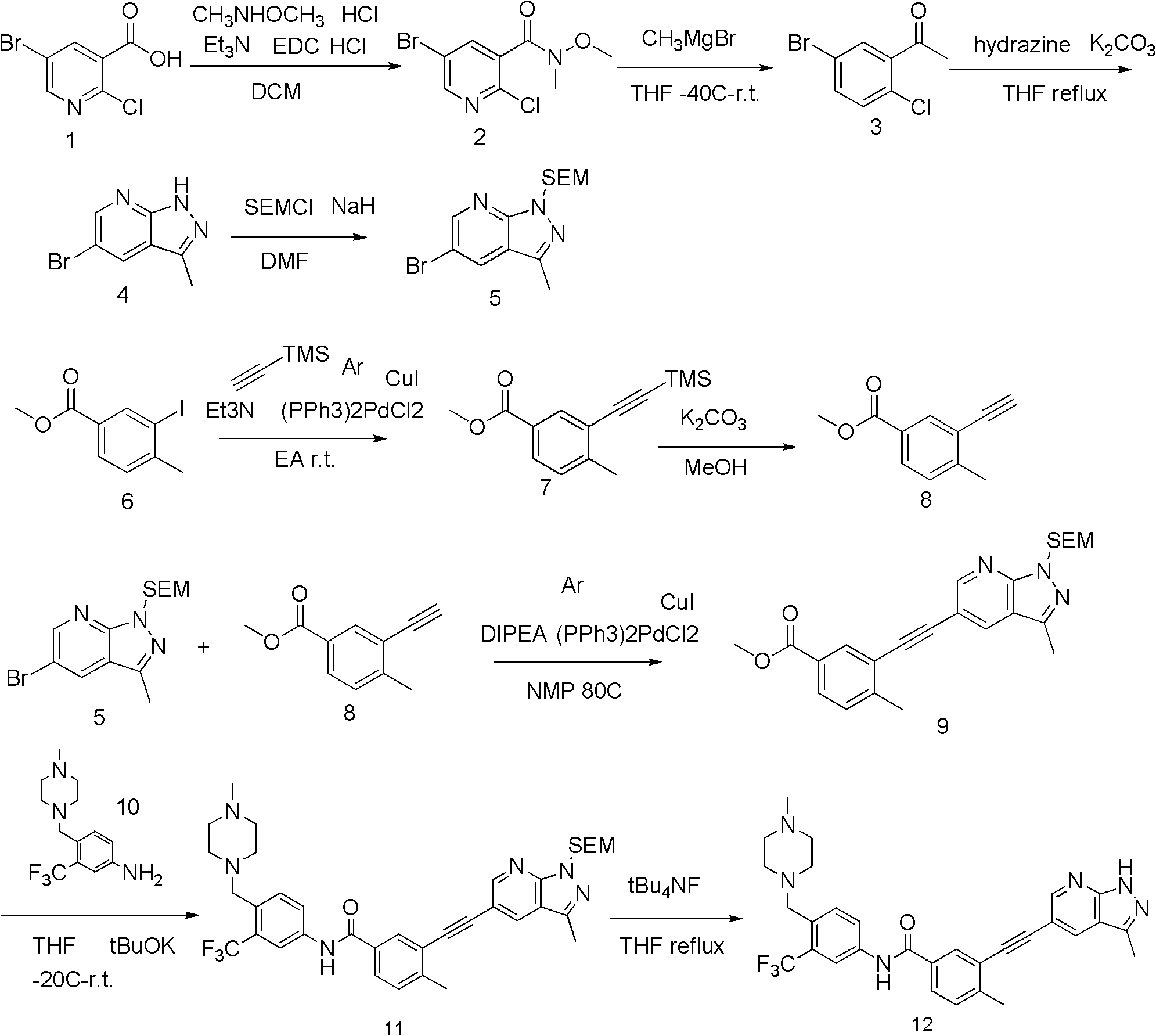
Pyrazolopyridines alkynylbenzene compound and medicinal composition and application
A technology of pyrazolopyridines and compounds, which is applied in drug combination, organic chemistry, antineoplastic drugs, etc., can solve the problem of huge quantity, and achieve the effects of high drugability, good pharmacokinetics and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 4-methyl-3-((3-methyl-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-N-(4-((4-methylpiperazine- 1-yl)methyl)-3-trifluoromethylphenyl)benzamide
[0064] 4-methyl-3-((3-methyl-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-N-(4-((4-methylpiperazin-1-yl)methyl)- 3-(trifluoromethyl)phenyl)benzamide
[0065]
[0066] step 1.
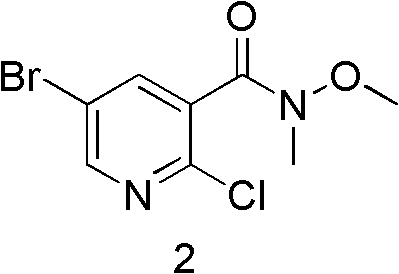
[0067] 5-Bromo-2-chloro-N-methoxy-N-methylnicotinamide
[0068] 5-bromo-2-chloro-N-methoxy-N-methylnicotinamide
[0069]
[0070] Dissolve 96g (294.07mmol) of 5-bromo-2-chloronicotinic acid in 1L of dichloromethane, and add 31.45g (323.48mmol) of N, O-dimethylhydroxylamine hydrochloride and 62g (323.48mmol) of EDC hydrochloride under stirring. mmol), finally dropwise added triethylamine 39.2g (388.17mmol), stirred overnight, the reaction solution was washed with water, washed with saturated brine, the organic phase was dried with anhydrous sodium sulfate, the solvent was spin-dried under reduced pressure, and column chromatography obtained 65g of a white s...
Embodiment 2
[0128] N-(3-(1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-4-methyl-3-((3-methyl-1H-pyrazolo[3,4 -b]pyridin-5-yl)ethynyl)benzamide
[0129] N-(3-(1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-4-methyl-3-((3-methyl-1H-pyrazolo[3,4-b]pyri din-5- yl)ethynyl)benzamide
[0130]
[0131] The synthetic method is as embodiment 1
[0132] 1 HNMR (400MHz, d-DMSO), δppm 13.49(s, 1H), 10.75(s, 1H), 8.69(d, J=2.0Hz, 1H), 8.52(d, J=2.0Hz, 1H), 8.34( s, 2H), 8.22(s, 2H), 7.95(d, J=8.0Hz, 1H), 7.79(s, 2H), 7.56(d, J=8.0Hz, 1H), 7.17(s, 1H), 2.60(s, 3H), 2.53(s, 3H).
[0133] MS (ESI), m / z: 501 (M + +H + ).
Embodiment 3
[0135] 4-methyl-N-(3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-3-((3-methyl-1H-pyrazole And[3,4-b]pyridin-5-yl)ethynyl)benzamide
[0136] 4-methyl-N-(3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-3-((3-methyl-1H-pyrazolo[3,4-b]pyridin -5-yl)ethynyl)benzamide
[0137]
[0138] The synthetic method is as embodiment 1
[0139] 1 HNMR (400MHz, d-DMSO), δppm 13.49(s, 1H), 10.72(s, 1H), 8.70(d, J=1.6Hz, 1H), 8.52(d, J=1.6Hz, 1H), 8.30( s, 1H), 8.21(s, 2H), 8.17(s, 2H), 7.95(m, 2H), 7.74(s, 2H), 7.56(d, J=8.0Hz, 1H), 7.49(s, 2H ), 2.60(s, 3H), 2.54(s, 3H), 2.19(s, 3H).
[0140] MS (ESI), m / z: 515 (M + +H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com