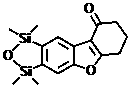
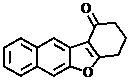
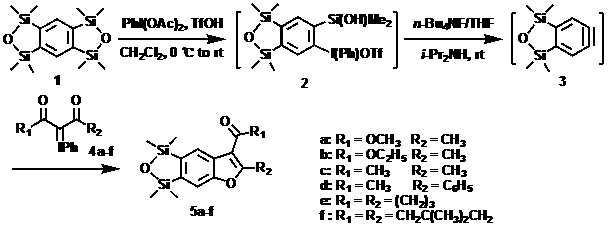
Benzofuran or naphthofuran derivative and preparation method thereof
A technology of furan derivatives and benzofuran, which is applied in the field of benzofuran or naphthofuran derivatives and their preparation, can solve the problems of long route, high catalyst price, limited expansion of substituted functional groups, etc., and achieve fast reaction speed and Mild conditions and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Iodophenyl diacetate (1.5-2.5 eq), trifluoromethanesulfonic acid (3.5-4.5 eq), benzobis(oxydisiloxane) ( 1 , 1.0 eq), diisopropylamine (3.0~4.0 eq), iodine ylide compound ( 4a , 4.0~5.0 eq), TBAF / THF (3.0~4.0 eq), the obtained benzofuran derivative is: 2-methyl-5,6-oxodisilyl-3-benzofurancarboxylic acid methyl ester ( 5a ), yield 83%, the structure of this compound is:
[0036]
[0037] Molecular formula: C 15 h 20 o 4 Si 2
[0038] Chinese name: Chinese name: 2-Methyl-5,6-oxodisilyl-3-benzofurancarboxylic acid methyl ester
[0039] English name: 2-Methyl-5,6-oxadisilole-3-benzofuran carboxylic acid, methyl ester
[0040] Molecular weight: 320.09
[0041] Appearance: white powder
[0042] H NMR (500 MHz, CDCl 3 ): δ 0.38 (s, 6H), 0.40 (s, 6H), 2.77 (s, 3H), 3.96 (s, 3H), 7.59 (s, 1H), 8.14 (s, 1H) ppm.
[0043] Carbon NMR (125 MHz, CDCl 3 ): δ 1.3, 1.4, 14.7, 51.6, 108.7, 113.1, 124.7, 127.8, 142.4, 143.7, 155.2, 164.3, 165.0 ppm.
[0044]...
Embodiment 2
[0045] Example 2: iodophenyl diacetate (1.5-2.5 eq), trifluoromethanesulfonic acid (3.5-4.5 eq), benzobis(oxydisiloxane) ( 1 , 1.0 eq), diisopropylamine (3.0~4.0 eq), iodine ylide compound ( 4b , 4.0~5.0 eq), TBAF / THF (3.0~4.0 eq), the obtained benzofuran derivative is: ethyl 2-methyl-5,6-oxodisilyl-3-benzofurancarboxylate ( 5b ), yield 91%, the structure of the compound is:
[0046]
[0047] Molecular formula: C 16 h 22 o 4 Si 2
[0048] Chinese name: ethyl 2-methyl-5,6-oxodisilyl-3-benzofurancarboxylate
[0049] English name: 2-Methyl-5,6-oxadisilole-3-benzofuran carboxylic acid, ethyl ester
[0050] Molecular weight: 334.11
[0051] Appearance: white powder
[0052] H NMR (500 MHz, CDCl 3 ): δ 0.39 (s, 6H), 0.40 (s, 6H), 1.45 (t, J = 7.0 Hz, 3H), 2.78 (s, 3H), 4.43 (q, J = 7.0 Hz, 2H), 7.60 (s, 1H), 8.18 (s, 1H) ppm.
[0053] Carbon NMR (125 MHz, CDCl 3 ): δ 1.35, 1.42, 14.6, 14.8, 60.5, 109.0, 113.1, 124.8, 128.0, 142.4, 143.7, 155.2, 164.0, 164.6 pp...
Embodiment 3
[0055] Example 3: Iodophenyl diacetate (1.5-2.5 eq), trifluoromethanesulfonic acid (3.5-4.5 eq), benzobis(oxydisiloxane) ( 1 , 1.0 eq), diisopropylamine (3.0~4.0 eq), iodine ylide compound ( 4c , 4.0~5.0 eq), TBAF / THF (3.0~4.0 eq), the obtained benzofuran derivatives are: 2-methyl-3-acetyl-5,6-oxodisilylbenzofuran ( 5c ), yield 85%, the structure of this compound is:
[0056]
[0057] Molecular formula: C 15 h 20 o 3 Si 2
[0058] Chinese name: 2-methyl-3-acetyl-5,6-oxodisilylbenzofuran
[0059] English name: 2-Methyl-3-acetyl-5,6-oxadisilole benzofuran
[0060] Molecular weight: 304.10
[0061] Appearance: white powder
[0062] H NMR (500 MHz, CDCl 3 ): δ 0.39 (s, 6H), 0.41 (s, 6H), 2.66 (s, 3H), 2.79 (s, 3H), 7.62 (s, 1H), 8.17 (s, 1H) ppm.
[0063] .Carbon NMR (125 MHz, CDCl 3 ): δ 1.3, 1.5, 15.7, 31.4, 113.3, 117.6, 124.5, 127.8, 142.8, 144.0, 155.1, 163.3, 194.3 ppm.
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com