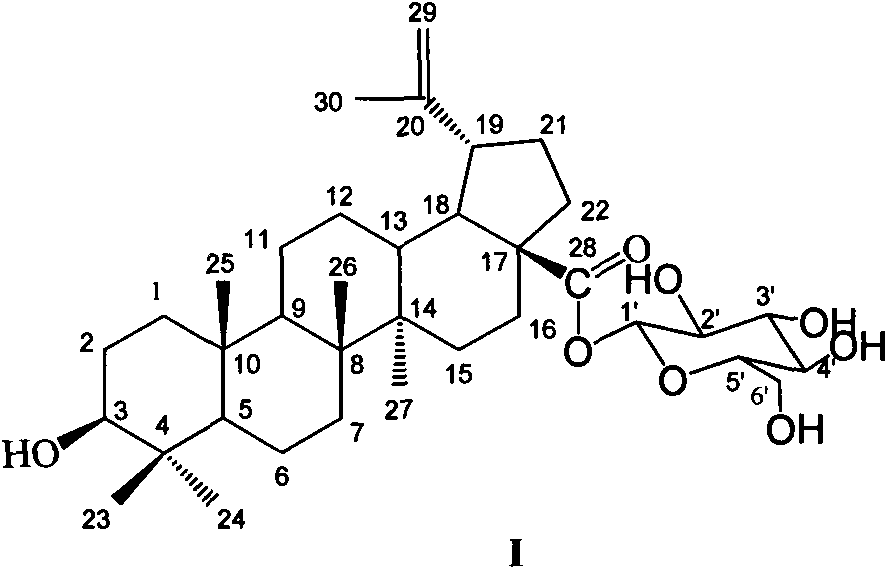
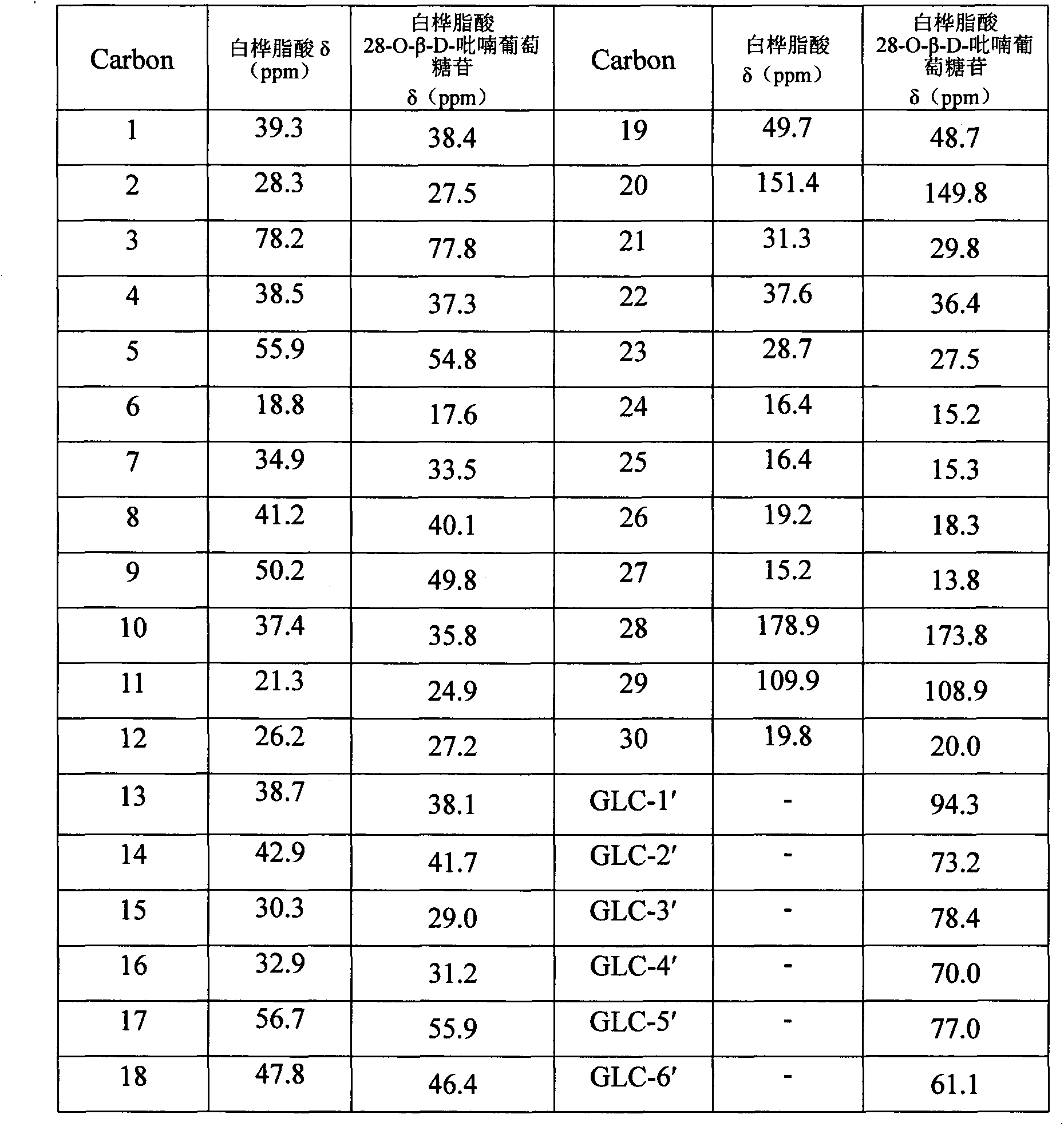
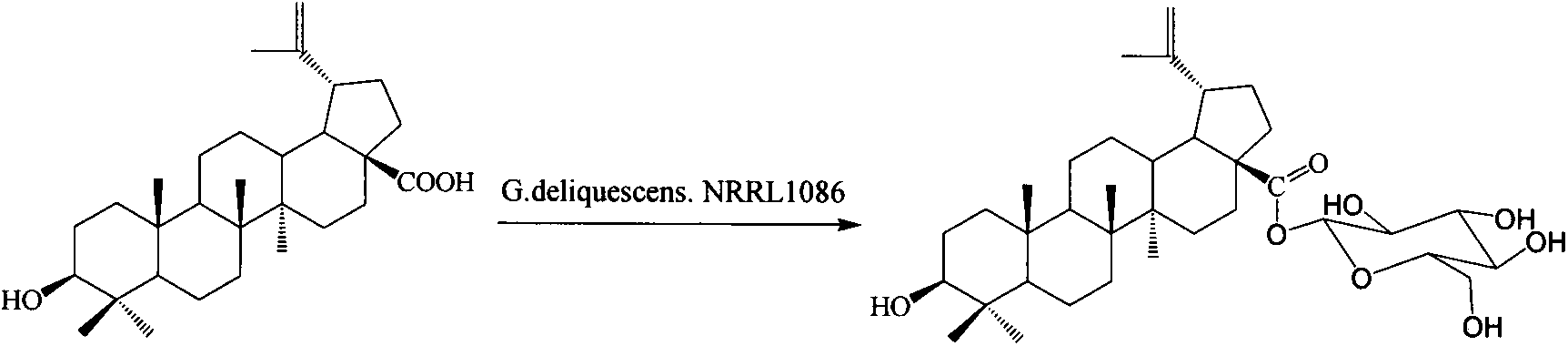
Preparation method of betulinic acid 28-O-beta-D-glucopyranoside and use of betulinic acid 28-O-beta-D-glucopyranoside
A technology of glucopyranoside and betulinic acid, applied in the field of biopharmaceuticals, can solve the problem of rare modification of betulinic acid, and achieve excellent anti-inflammatory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of betulinic acid 28-O-β-D-glucopyranoside:
[0058] Dissolve 200 mg of betulinic acid as a substrate in 20 mL of ethanol; prepare 100 mL of 0.5 g / mL glucose solution, and sterilize (conditions are the same as PDA); the strain NRRL1086 is inoculated into liquid PDA medium from a solid slope at 4°C (the medium is 100 mL 20% Potato decoction contains KH 2 PO 4 0.3 g; MgSO 4 0.75g; glucose 10g; vitamin B10.01g, the liquid medium was divided into 150mL Erlenmeyer flasks, each bottle containing 30mL), and cultured in a shaker for 24 hours (28°C, 180r / m), that is, the seeds liquid; the seed liquid was transferred to the liquid PDA medium (the composition of the medium was the same as before) under aseptic conditions, and continued to cultivate in the shaker for 24 hours. Add 1mL betulinic acid ethanol solution and 3mL sucrose solution to each bottle, and stop the reaction after culturing for 144 hours. The fermentation broth was extracted 6 times with an equal...
Embodiment 2
[0061] tablet
[0062] Take 100 g of betulinic acid 28-O-β-D-glucopyranoside prepared in Example 1, mix with 50 g of starch and 50 g of dextrin, use an appropriate amount of 30% ethanol as a wetting agent to make a soft material, and granulate by conventional methods , add an appropriate amount of magnesium stearate and mix to make a tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com