Oncolytic adenoviral vectors coding for monoclonal anti-CTLA-4 antibodies
An oncolytic adenovirus, human monoclonal antibody technology, used in life sciences and medicine to achieve safe cancer treatment tools and improve the effect of cancer therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
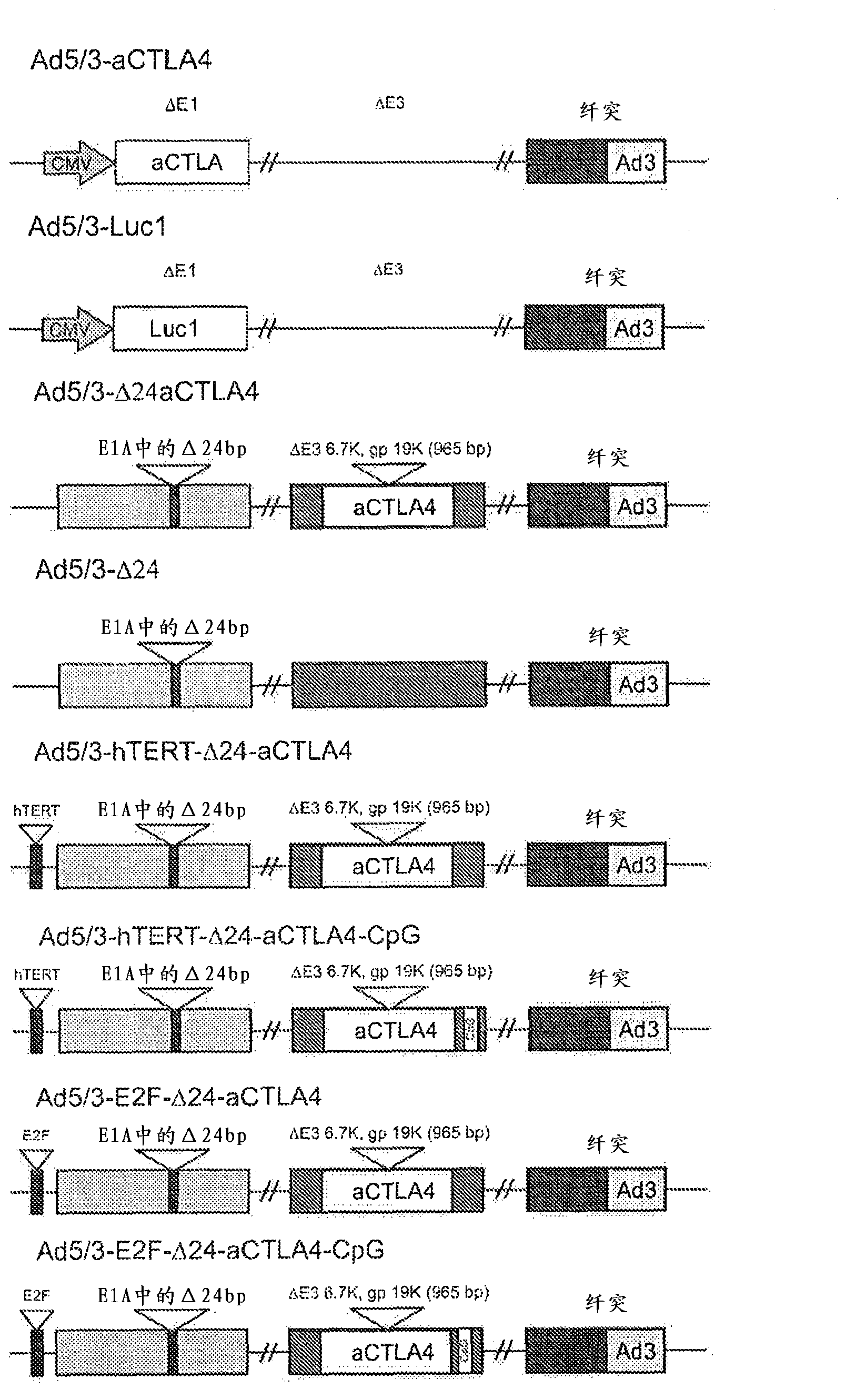
[0158] Example 1. Construction of adenovirus
[0159] Generation of chimeric adenoviruses with cDNA sequences encoding IgG2 type anti-CTLA4 mAbs ( figure 1 ). The coding sequence of the anti-CTLA4 mAb was introduced into the 6.7K / gp19K deletion of adenovirus E3A to produce replication-competent adenoviruses Ad5 / 3-Δ24aCTLA4 (SEQ ID.NO:1), Ad5 / 3-hTERT-Δ24aCTLA4 (SEQ ID.NO:2), Ad5 / 3-hTERT-Δ24aCTLA4-CpG (SEQ ID.NO:3), Ad5 / 3-E2F-Δ24aCTLA4 (SEQ ID.NO:4) and Ad5 / 3-E2F-Δ24aCTLA4-CpG (SEQ ID. NO: 5), or introduce a deletion at E1 driven by a CMV promoter to generate a replication-defective adenovirus Ad5 / 3-aCTLA4 (SEQ ID. NO: 6).
[0160] Using standard adenovirus preparation techniques (Kanerva A, et al., Mol Ther 2002; 5:695-704; Bauerschmitz GJ et al., Mol Ther 2006; 14:164-74; Kanerva A and Hemminki A., Int J Cancer 2004; 110:475-74; 80; Volk AL, et al., Cancer Biol Ther 2003;2:511-5) Generation and amplification of oncolytic adenovirus. Briefly, E1 or E3 shuttle vectors with t...
Embodiment 2
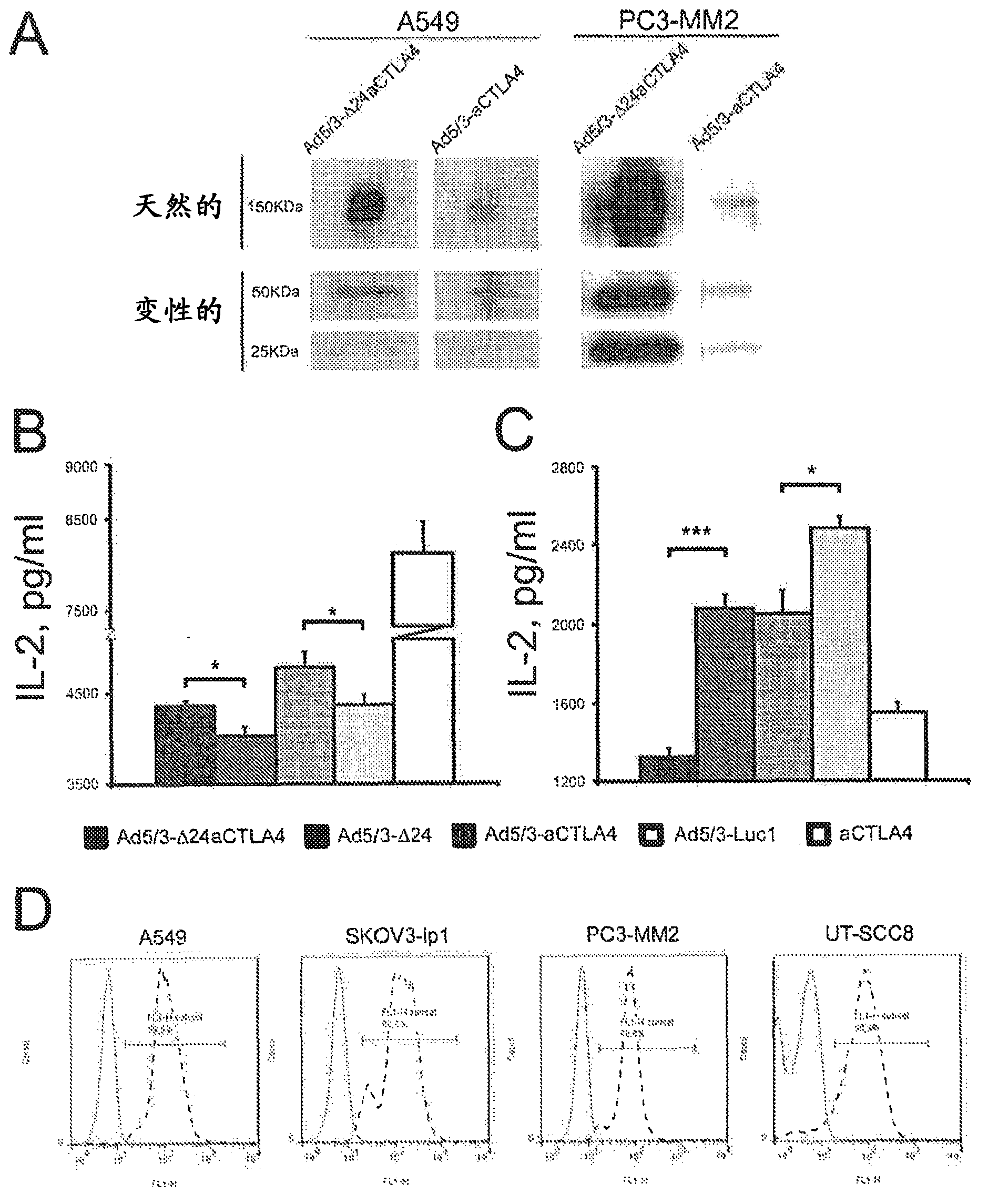
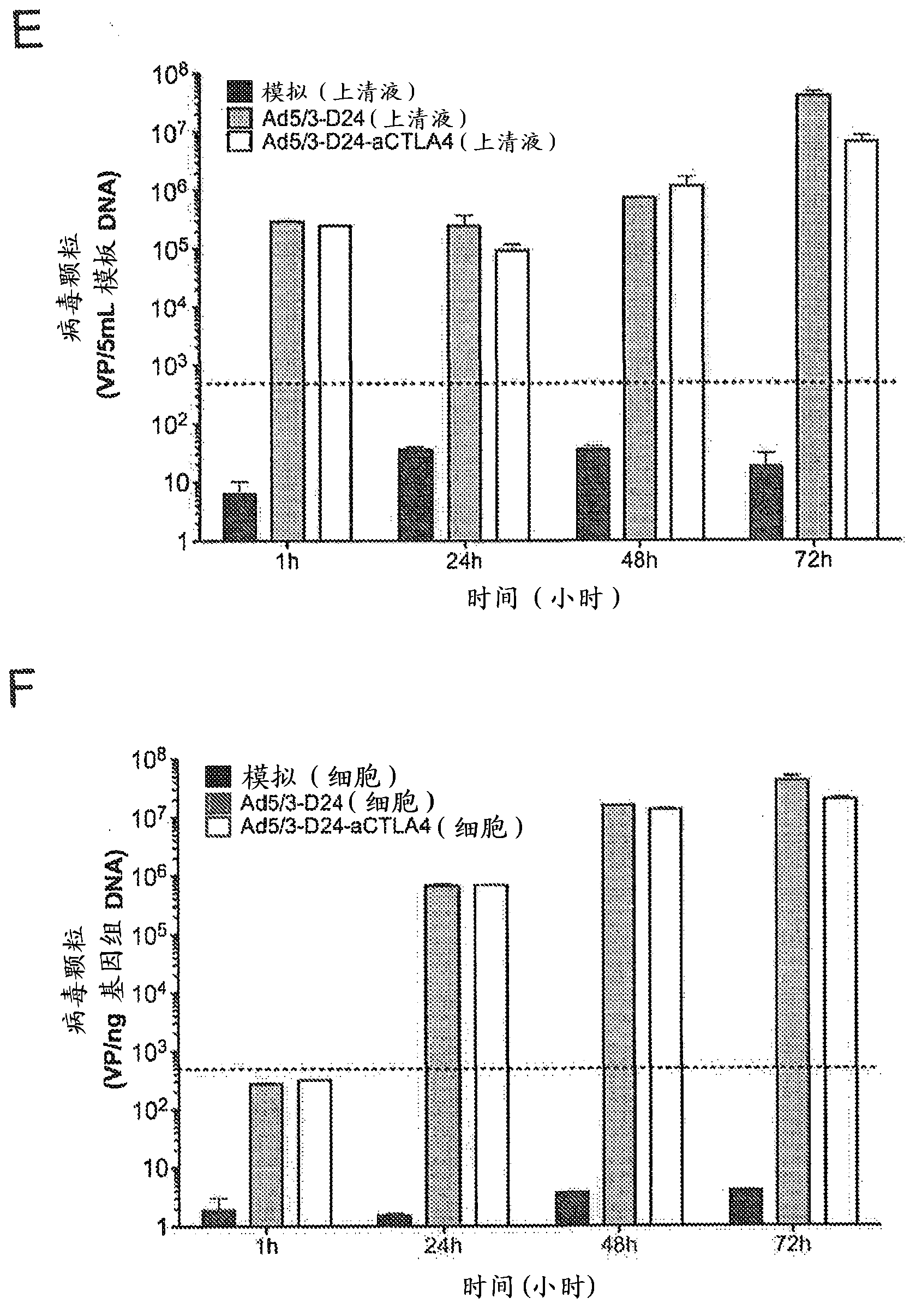
[0165] Example 2. In vitro expression and functionality of the constructed adenovirus
[0166] Western blot analysis was used to confirm that the constructed adenovirus expressed anti-CTLA4 mAb. A549 or PC3-MM2 tumor cells were infected with the constructed Ad5 / 3-Δ24aCTLA4 or Ad5 / 3-aCTLA4 at 10 viral particles (VP) / cell. After 48 hours, the supernatant of virus-infected cells was filtered with a 0.02 μm filter (Anotop, Whatman, England), and 15 μL was used for 7.5% SDS-polyacrylamide gel electrophoresis (PAGE ), and then transferred to nitrocellulose membrane. Membranes were incubated with goat anti-human IgG (heavy and light chains) (AbD serotec, MorphoSys, Germany), washed, and subsequently incubated with a secondary antibody conjugated to horseradish peroxidase (Dako, Denmark) . Signal detection was performed using enhanced chemiluminescence (GE Healthcare, Amersham, UK).
[0167] In Western blot, Ad5 / 3-Δ24aCTLA4 and Ad5 / 3-aCTLA4 expressed the expected anti-CTLA4 mAb of...
Embodiment 3
[0174] Example 3. CTLA-4 Expression in Tumor Cell Lines and Low-Passage Tumor Explants
[0175] Since it has been reported that almost 90% of tumor cell lines express CTLA-4 and that anti-CTLA-4 mAbs may have direct antitumor activity (13), it is important to study the expression of CTLA-4 in tumor cell lines, including HNSCC using low-passage tumor explants. Is this also the case in the body.
[0176] Indirect immunofluorescence was performed in the low passage tumor cell culture UT-SCC8 or the tumor cell lines A549, SKOV3-ip1 and PC3-MM2 to analyze surface CTLA-4. Briefly, mouse anti-human CTLA-4 mAb (BD Pharmingen TM, Europe) was used as the primary antibody to incubate the cell pellet for 30 minutes, and then incubated with Alexa at 4°C 488 donkey anti-mouse IgG (Invitrogen) was incubated as secondary antibody for an additional 30 minutes. In the LSR flow cytometer (BDPharmingen TM , Europe) to measure fluorescence intensity. Count at least 40,000 cells / sample. Usin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com