Oncolytic adenoviral vectors and methods and uses related thereto
A technology of oncolytic adenovirus and adenovirus, applied in the field of life science and medicine, to improve the effect of cancer therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
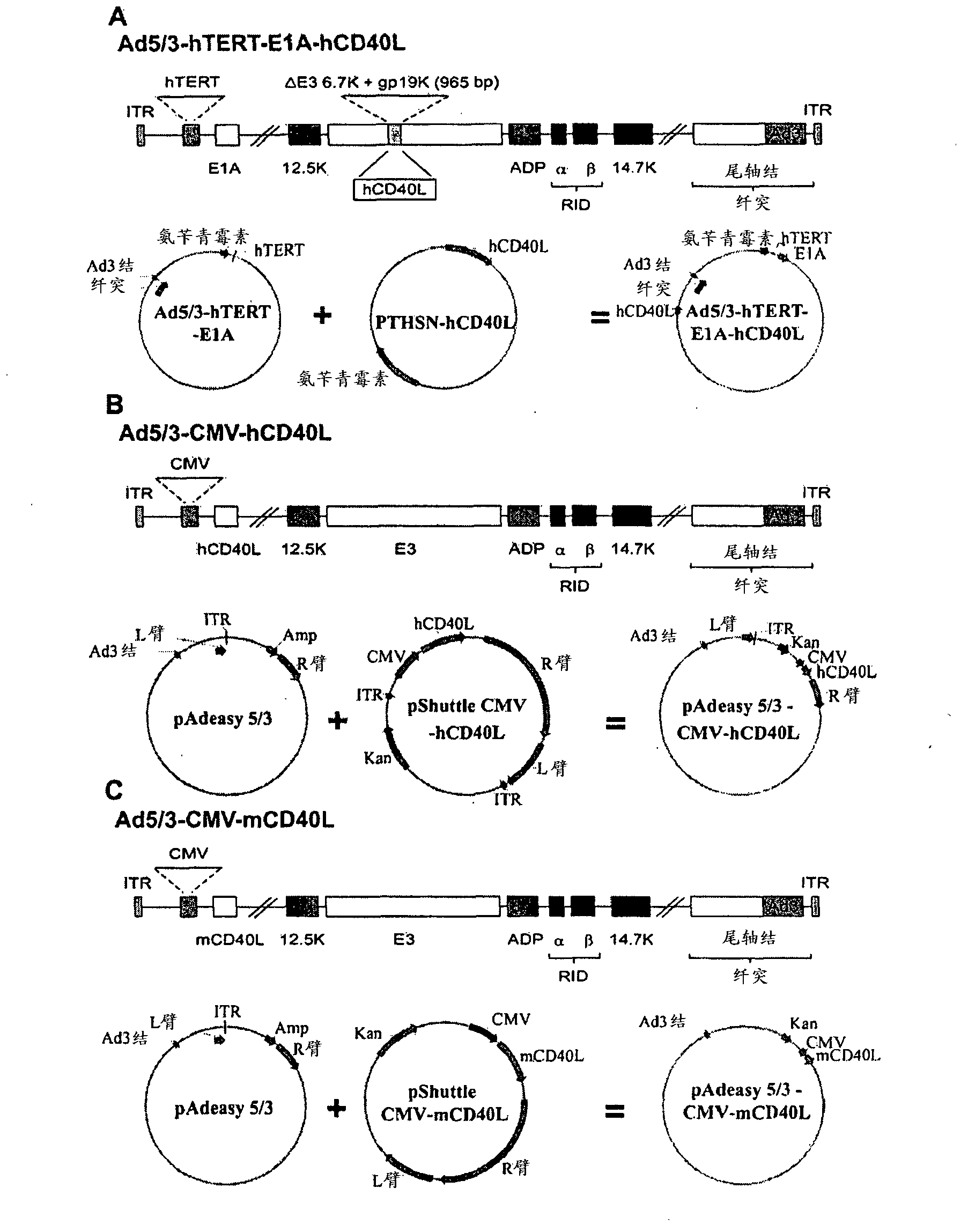
[0136] Example 1. Cloning of Ad5 / 3-hTERT-E1A-hCD40L, Ad5 / 3-CMV-hCD40L and Ad5 / 3-CMV-mCD40L
[0137] Using standard adenovirus preparation techniques (Kanerva A, et al., Mol Ther 2002; 5:695-704; Bauerschmitz GJ, et al., Mol Ther 2006; 14:164-74; Kanerva A and Hemminki A., Int J Cancer 2004; 110: 475-80; Volk AL, et al., Cancer Biol Ther 2003; 2:511-5) produced and amplified Ad5 / 3-hTERT-E1A-hCD40L (SEQ ID. NO:5). Briefly, using specific primers (forward primer: TTTAACATCTCTCCCTCTGTGATT; SEQ ID NO: 3 and reverse primer: TATAAATGGAGCTTGACTCGAAG; SEQ ID NO: 4) (characterized by the insertion of specific restriction sites SunI / MunI) by polymerization Human CD40L cDNA was amplified by enzyme chain reaction (PCR) (a kind gift from Prof Eliopoulos, University of Crete, Heraklion, Greece). The PCR amplified product was subsequently subcloned into pTHSN (Kanerva A., et al., Gene Ther 2005; 12:87-94), which was then combined with pAd5 / 3-hTERT-E1A (Bauerschmitz GJ, et al., Cancer Res 200...
Embodiment 2
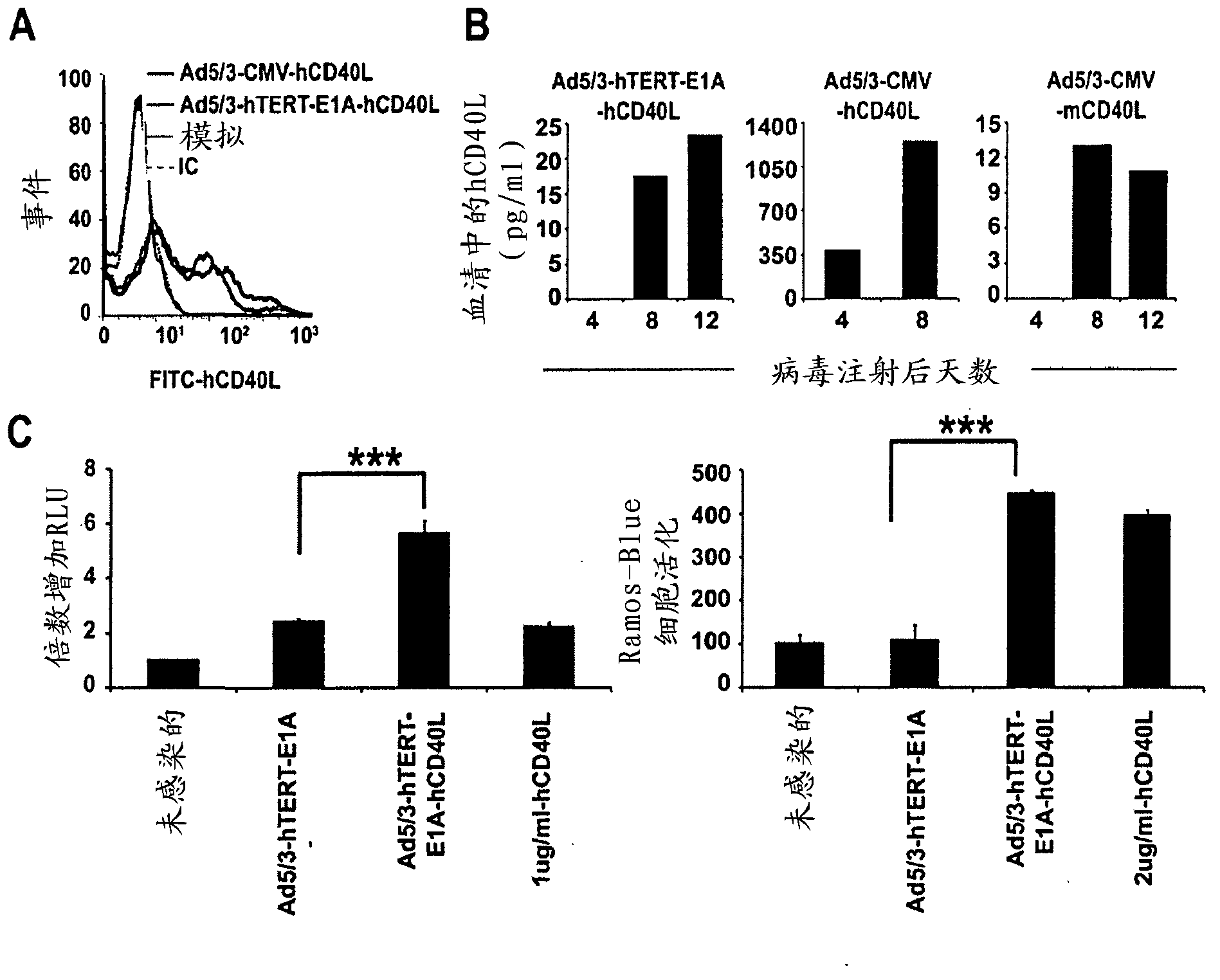
[0141] Example 2. Expression and functionality of constructed adenoviruses: in vitro and in vivo
[0142] hCD40L expression was studied using flow cytometry and enzyme-linked immunosorbent assay (ELISA). For flow cytometry analysis, human embryonic kidneys were infected with Ad5 / 3-hTERT-E1A-hCD40L or Ad5 / 3-CMV-hCD40L at 10 VP / cell in growth medium containing 2% fetal calf serum (FCS) 293 cells. Control cells were treated with 2% Dulbecco's Modified Eagle's Medium (DMEM) alone (mock test). After 24 hours, cells were stained with hCD40L-FITC (555699, BD Biosciences Pharmingen Franklin Lakes, NJ) antibody for 30 minutes, or stained with an isotype control (IC) to measure the expression from cell autofluorescence and non-antigen-specific binding. Bottom fluorescence. Flow cytometry analysis was performed on a BDLSR (BD Biosciences, Franklin Lakes, NJ).
[0143] For ELISA analysis, A549 xenografts and syngeneic MB49 tumors were induced and treated with Ad5 / 3-hTERT-E1A-hCD40L, A...
Embodiment 3
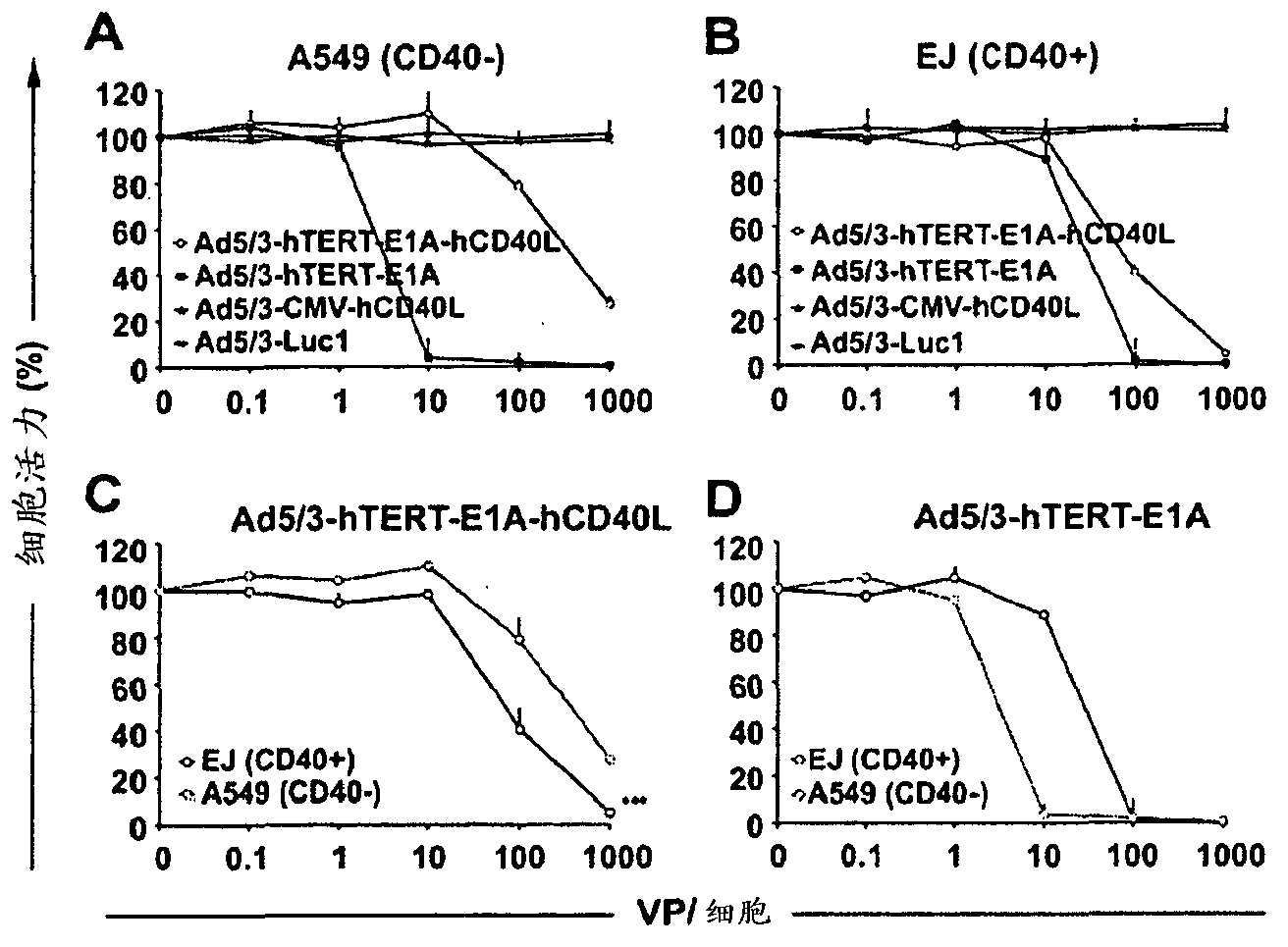
[0151] Example 3. In vitro evaluation of the oncolytic efficacy of the constructed adenovirus
[0152]To evaluate the oncolytic potency of the constructed adenovirus, EJ (CD40+) and A549 (CD40-) cell lines were used. In the cell viability assay, different concentrations (0.1, 1, 10, 100, 1000 VP / cell) of Ad5 / 3-hTERT-E1A-hCD40L, Ad5 / 3-CMV-hCD40L and their controls suspended in 2% DMEM were used Viruses Ad5 / 3-hTERT-E1A and Ad5 / 3-Luc1 infect cells in 96-well plates. After 1 hour, cells were washed and incubated for 7 days in growth medium containing 5% FCS. Cell viability was subsequently analyzed using the MTS assay (Cell Titer96A Queous One Solution Proliferation Assay, Promega).
[0153] For Ad5 / 3-hTERT-E1A-hCD40L, complete cell killing was seen in the EJ (CD40+) cell line at 1000 viral particles / cell (VP / cell) ( image 3 b). In the A549(CD40-) cell line, the oncolytic potency of Ad5 / 3-hTERT-E1A-hCD40L was slower than that of the control virus Ad5 / 3-hTERT-E1A( image 3 a)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com