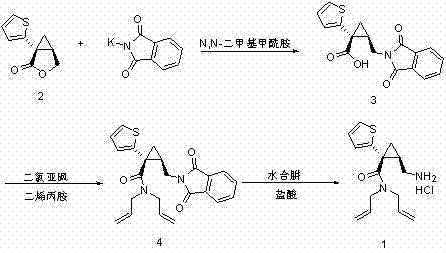
Preparation method of N,N-diallyl-(1R,2R)-2-aminomethyl-1-(2-thienyl)cyclopropanecarboxamide hydrochloride
A cyclopropane amide and diallyl technology, which is applied in the field of medicine and pharmaceutical preparation, can solve the problems of industrialized production and the inability of cyclopropane amide hydrochloride, and achieve the advantages of improving yield, improving safety and avoiding product loss. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Dissolve 1.82 kg of 1-(2-thiophene) cyclopropane lactone (Formula 2) and 2.67 kg of anhydrous aluminum trichloride in 20 liters of dichloromethane, cool the reaction system to -10°C, and add 3.89 kg of diene dropwise Propylamine. After the reaction system was stirred for 30 minutes, 11 liters of hydrochloric acid was added to the reaction system, and the organic layer was separated, and then washed with 10 liters of hydrochloric acid and 10 liters of water respectively, dried with anhydrous sodium sulfate for 0.5 hours, filtered to remove solids, and the filtrate was reduced. Concentrate under pressure to remove the solvent. Finally, the volume of the mixed liquid after rotary evaporation is 27 liters, and a black oily N,N-diallyl-(1R, 2R)-2-hydroxymethyl containing dichloromethane is obtained. 1-(2-thiophene)cyclopropane (formula 5) solution, this mixed solution is directly put into the next reaction without further purification. LC-MS characterization of purity> 99%, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com