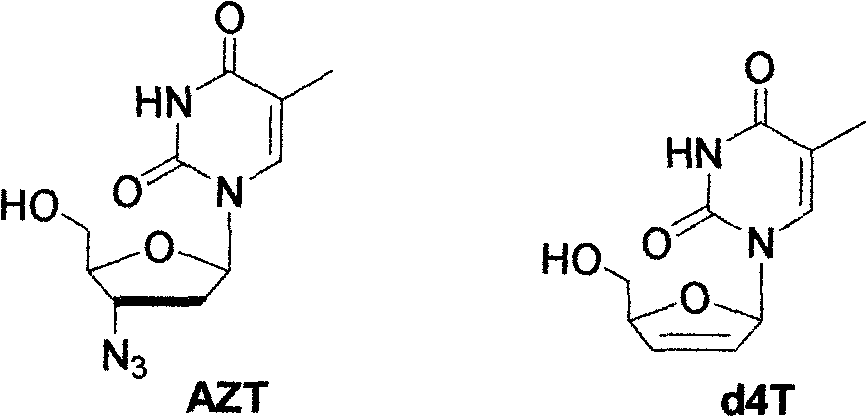
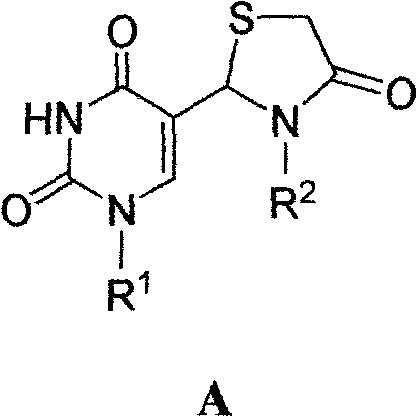
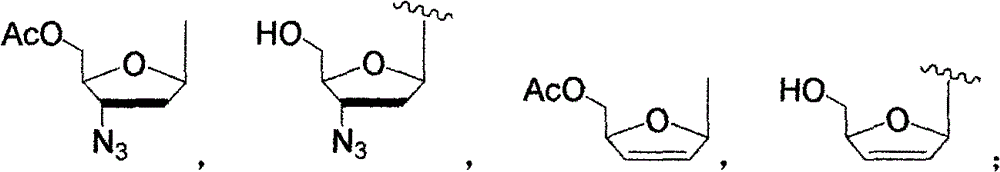
5-substituted pyrimidine nucleoside-thiazolinone hybrid with anti-HIV activity and preparation method thereof
A formyl pyrimidine nucleoside compound, pyrimidine nucleoside technology, applied in the directions of organic active ingredients, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve problems such as unreported, achieve simple preparation process, synthetic The effect of short route and significant anti-HIV activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1.5-(4-oxo-3-(4-methylpyridin-2-yl)-thiazoline-2-yl)-3'-azido-2', 3'-dideoxy-5' -Synthesis of acetoxyuridine (a)
[0028] Add 5-formyl-3'-azido-2', 3'-dideoxy-5'-acetoxyuridine (0.323g, 1mmol), 4-picoline-2 -Amine (0.108g, 1mmol) and thioglycolic acid (0.184g, 2mmol) and [bmim]PF6 (2mL), the reaction was stirred at 80°C until the end of the reaction (TLC follow the reaction). After the reaction, the reaction system was cooled to room temperature. Extracted three times with ethyl acetate (5 mL×3), combined the ethyl acetate phases, and then washed successively with 5% HCl, 5% NaHCO3 solution, and saturated NaCl solution. The organic phase was collected, dried by adding anhydrous Na2SO4, and then separated by column chromatography to obtain the target product a (0.297g), with a yield of 61%.
[0029] The structural formula of target product a:
[0030]
[0031] 1 HNMR (400MHz, CDCl 3 )δ: 2.15-2.46 (m, 8H), 3.65-3.70 (m, 1H), 4.02-4.35 (m, 5H), 5.98-6.02 (...
Embodiment 2
[0032] Example 2.5-(4-oxo-3-(3-bromophenyl)thiazoline-2-yl)-3'-azido-2',3'-dideoxyuridine (b) synthesis
[0033] Add 5-formyl-3'-azido-2',3'-dideoxyuridine (0.281g, 1mmol), 3-bromoaniline (0.172g, 1mmol) and thioglycolic acid ( 0.184g, 2mmol) and toluene (20mL), reflux reaction until the end of the reaction (TLC tracking reaction). After the reaction was completed, the solvent was evaporated, and the residue was dissolved in ethyl acetate, followed by 5% HCl, 5% NaHCO 3 solution and saturated NaCl solution. Collect the organic phase and add anhydrous Na 2 SO 4 Drying, followed by column chromatography to obtain target product b (0.295g), yield 58%.
[0034] The structural formula of target product b:
[0035]
[0036] 1 HNMR (400MHz, CDCl 3 )δ: 2.28-2.43(m, 2H), 3.54-4.28(m, 7H), 6.04-6.22(m, 2H), 7.20-7.62(m, 4H), 8.23-8.25(m, 1H), 10.04( brs, 1H). 13 CNMR (100MHz, CDCl 3)δ: 30.9, 33.9, 37.7, 38.3, 41.4, 59.6, 60.5, 60.7, 62.7, 63.4, 82.2, 82.7, 86.6, 86.9, 102....
Embodiment 3
[0037] Example 3.5-(4-oxo-3-phenylthiazolin-2-yl)-2',3'-dideoxy-2',3'-didehydro-5'-acetoxyuracil nucleus Synthesis of Glycoside (c)
[0038] In a reaction flask, 5-formyl-2′,3′-dideoxy-2′,3′-didehydro-5′-acetoxyuridine (1.12 g, 4 mmol) was dissolved in tetrahydrofuran (15 mL ), then add aniline (0.186g, 2mmol) and mercaptoacetic acid (0.552g, 6mmol) under ice-water bath conditions, and stir until the reaction is complete (TLC tracking monitoring). The solvent was evaporated, and the residue was dissolved in ethyl acetate, followed by 5% HCl, 5% NaHCO 3 solution, saturated NaCl solution to wash, collect the organic phase, add anhydrous NaCl 2 SO 4 Drying, followed by column chromatography to obtain target product c (0.29g), yield 67%.
[0039] The structural formula of target product c:
[0040]
[0041] 1 HNMR (400MHz, CDCl 3 )δ: 2.05-2.07(m, 3H), 3.72-4.22(m, 4H), 5.78-5.82(m, 1H), 5.94(s, 1H), 6.20-6.25(m, 1H), 6.78-6.82( m, 1H), 7.22-7.38 (m, 7H), 9.42 (brs, 1H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com