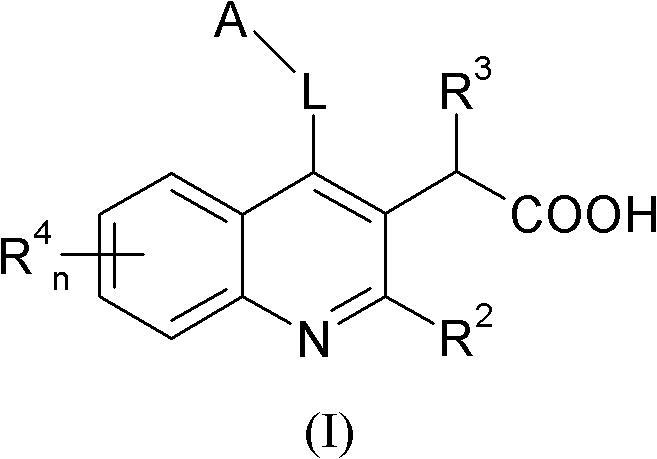
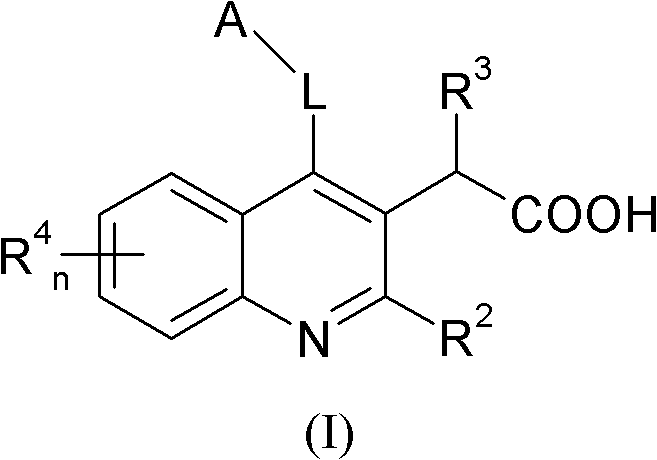
Intergrase inhibitor
A compound, alkyl technology, applied in the field of inhibitors that inhibit HIV replication, HIV treatment, and integrase allosteric inhibitors, which can solve the problems of crystal form stability, solubility lipophilicity, CYP inhibition liver microsome stability and plasma stability sexual insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Example 1 discloses a method for synthesizing a compound of formula (I).
[0187]
[0188]Condensation of methyl-2-aminobenzoate derivative (1a) with ethyl-3-ethoxy-2-enoate affords the imine, which then undergoes cyclization to yield quinoline 1b. A variety of reaction conditions can be employed to affect this condensation cyclization reaction. After that with SOCl 2 or POCl 3 Workup was performed to convert the phenol in 1b to the chloride. The chlorine in 1c is then replaced by an addition elimination reaction with the appropriate amine (benzylamine in Scheme 1) with heating in a suitable solvent.
[0189] The ester group in 1d is then reduced to the alcohol group using a suitable reducing agent such as, but not limited to, DIBAL. Reduction of the ester in 1d can also be carried out using other reducing conditions. Then use a method suitable for the oxidation of ethanol to acetaldehyde, such as DMSO oxidation conditions (such as SO 3 -pyridine), or with Mn...
Embodiment 2
[0192] Another synthetic method of 2C4 amine-substituted quinoline.
[0193]
[0194] Another method for the synthesis of compounds of formula (I) disclosed in Example 2. Condensation of methyl-2-aminobenzoate derivative (2a) with ethyl-3-ethoxy-2-enoate affords the imine, which then undergoes cyclization to yield quinoline 2b. Various reaction conditions can be used to influence this condensation cyclization reaction according to the relevant methods in this technical field. After that with SOCl 2 or POCl 3 Workup was performed to convert the phenol in 2b to chloride. The chlorine in 2c is then converted to iodine using NaI in a suitable solvent such as acetonitrile or acetone to give 2d. The ester group in 2d is then reduced to the alcohol group using a suitable reducing agent such as, but not limited to, DIBAL. The ester in 2d can also be reduced using other conditions suitable for ester reduction. Then use a method suitable for oxidation of alcohol groups to aceta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com