Method for preparing Ni2P catalyst
A technology of catalyst and inert gas, which is applied in the field of preparation of nickel phosphide catalyst and can solve problems such as limiting practical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
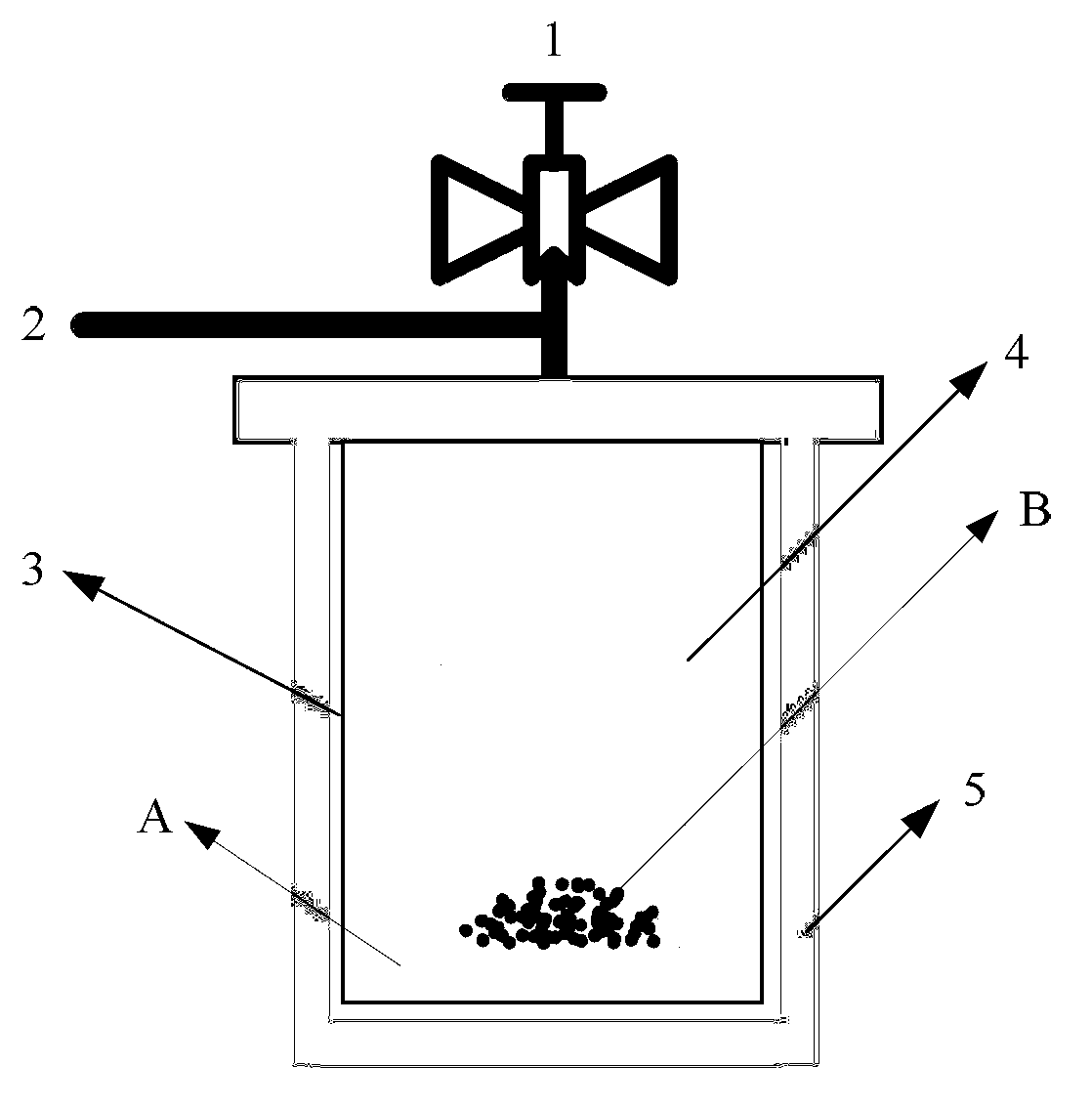
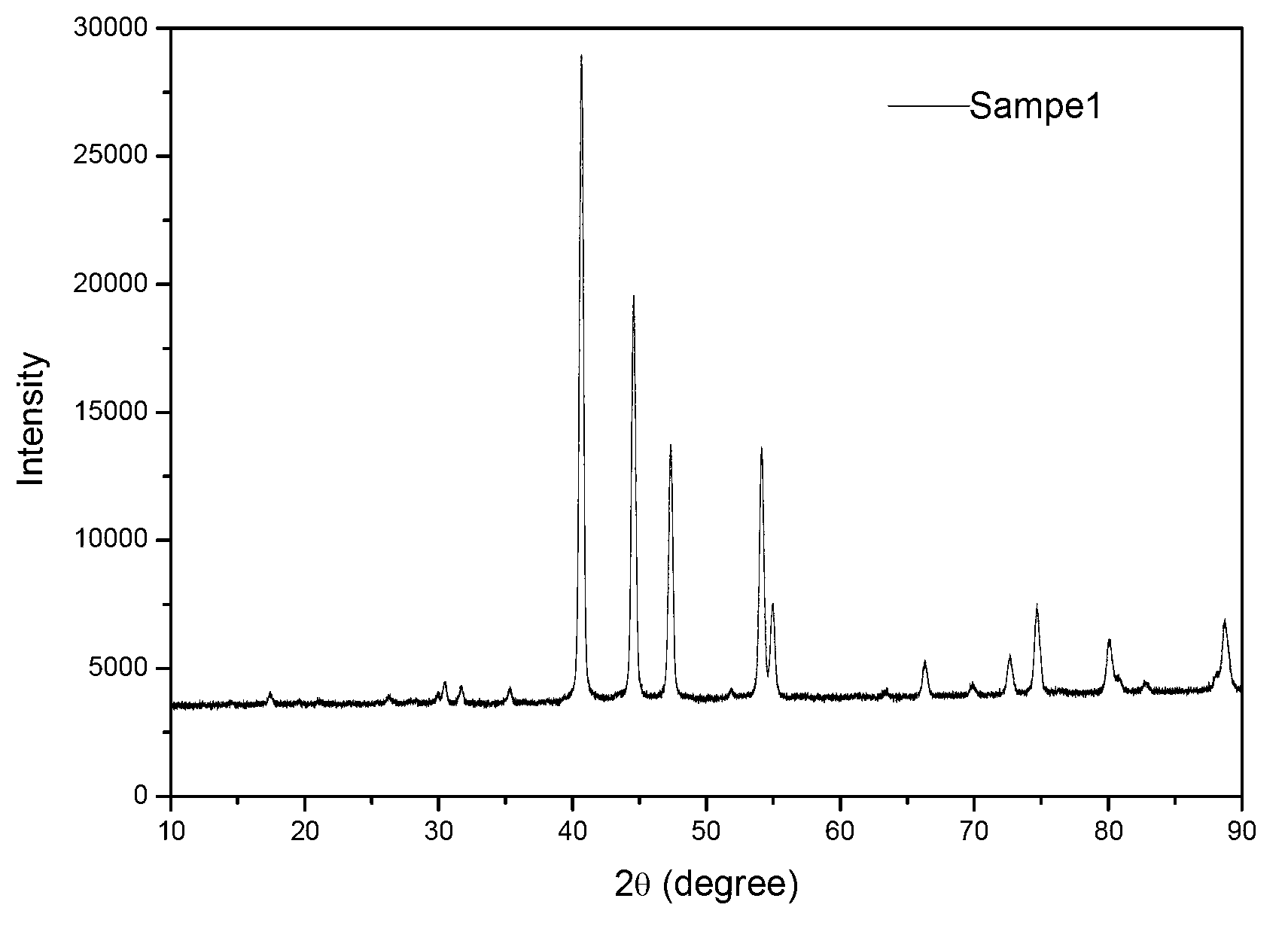
[0027] Take 2g NaH 2 PO 2 .H 2 O placed in a closed reactor (such as figure 1 Shown) the outer reaction bed layer, weigh the metal nickel powder with a P / Ni molar ratio of 3 and place it in the inner reaction bed layer of the reactor. The reactor is fed with N 2 After inflating, vacuumize again, and after repeating 3 times of ventilating, the reactor is fed with normal pressure N 2 Gas, under airtight conditions, heated up to 250°C at a rate of 5°C / min, reacted at constant temperature for 1h, cooled to room temperature, pumped vacuum and passed N 2 After replacing the reaction gas with gas, the sample located in the reaction bed inside the reactor is the prepared non-supported Ni 2 P catalyst, represented by Sample1, the XRD spectrum of Sample1 is shown in figure 2 .
Embodiment 2
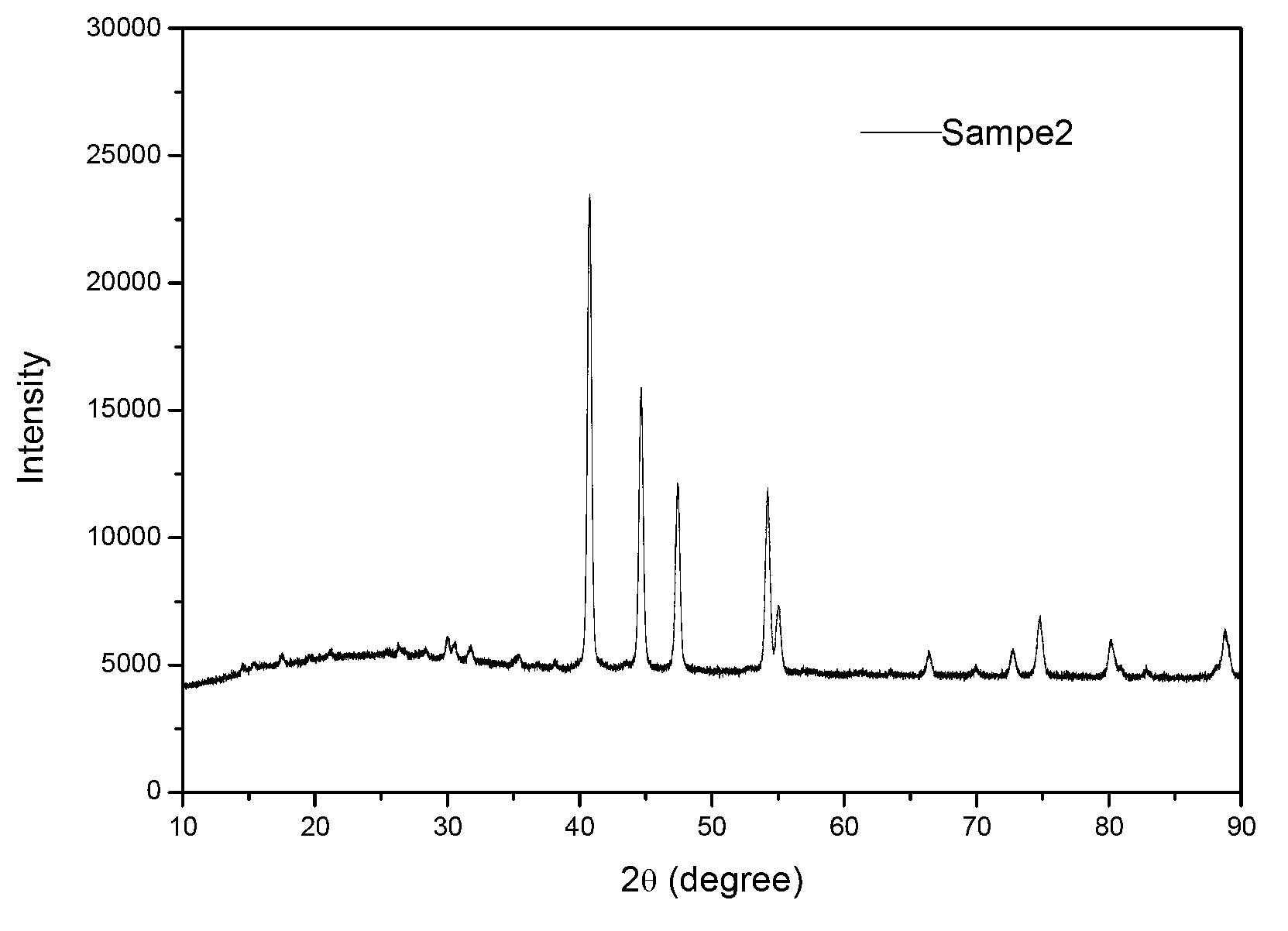
[0029] Take 2g NaH 2 PO 2 .H 2 O is placed in the external reaction bed of the closed reactor, and the metal nickel powder with a P / Ni molar ratio of 2 is weighed and placed in the internal reaction bed of the reactor. After the reactor was fed with Ar gas, it was evacuated again, and after repeated 3 times of gas exchange, the reactor was fed with Ar gas at normal pressure. At room temperature, after the reaction gas was replaced by Ar gas under vacuum, the sample located in the reaction bed inside the reactor was the prepared non-supported Ni 2 P catalyst, represented by Sample2, the XRD spectrum of Sample2 is shown in image 3 .
Embodiment 3
[0031] Take 2g KH 2 PO 2 .H 2 O is placed in the external reaction bed of the closed reactor, and the metal nickel powder with a P / Ni molar ratio of 4 is weighed and placed in the internal reaction bed of the reactor. After feeding He gas into the reactor, vacuumize the reactor again. After repeated ventilation for 3 times, vacuumize the reactor. After replacing the reaction gas with He gas, the sample located in the reaction bed inside the reactor is the prepared non-supported Ni 2 P catalyst, represented by Sample3, the XRD spectrum of Sample3 is shown in Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com