Anthranilamide compound as well as preparation method and application thereof
A technology of anthranilamide and anthranilamide, which is applied in the field of anthranilamide compounds and their preparation, can solve problems such as poor effect, and achieve the effects of low cost, simple preparation method and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
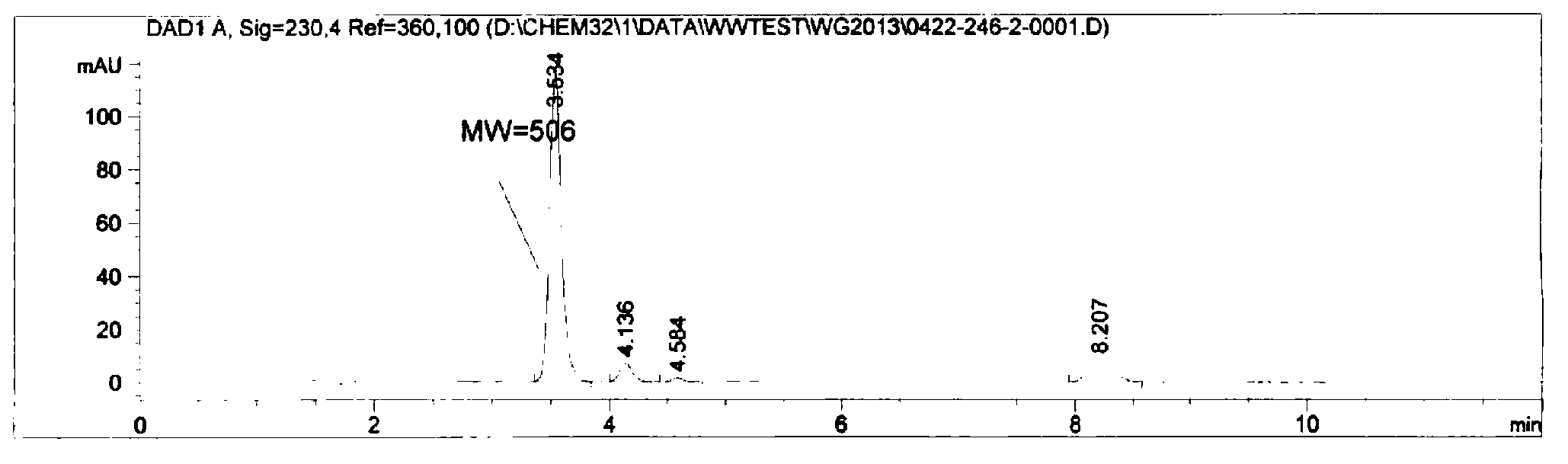
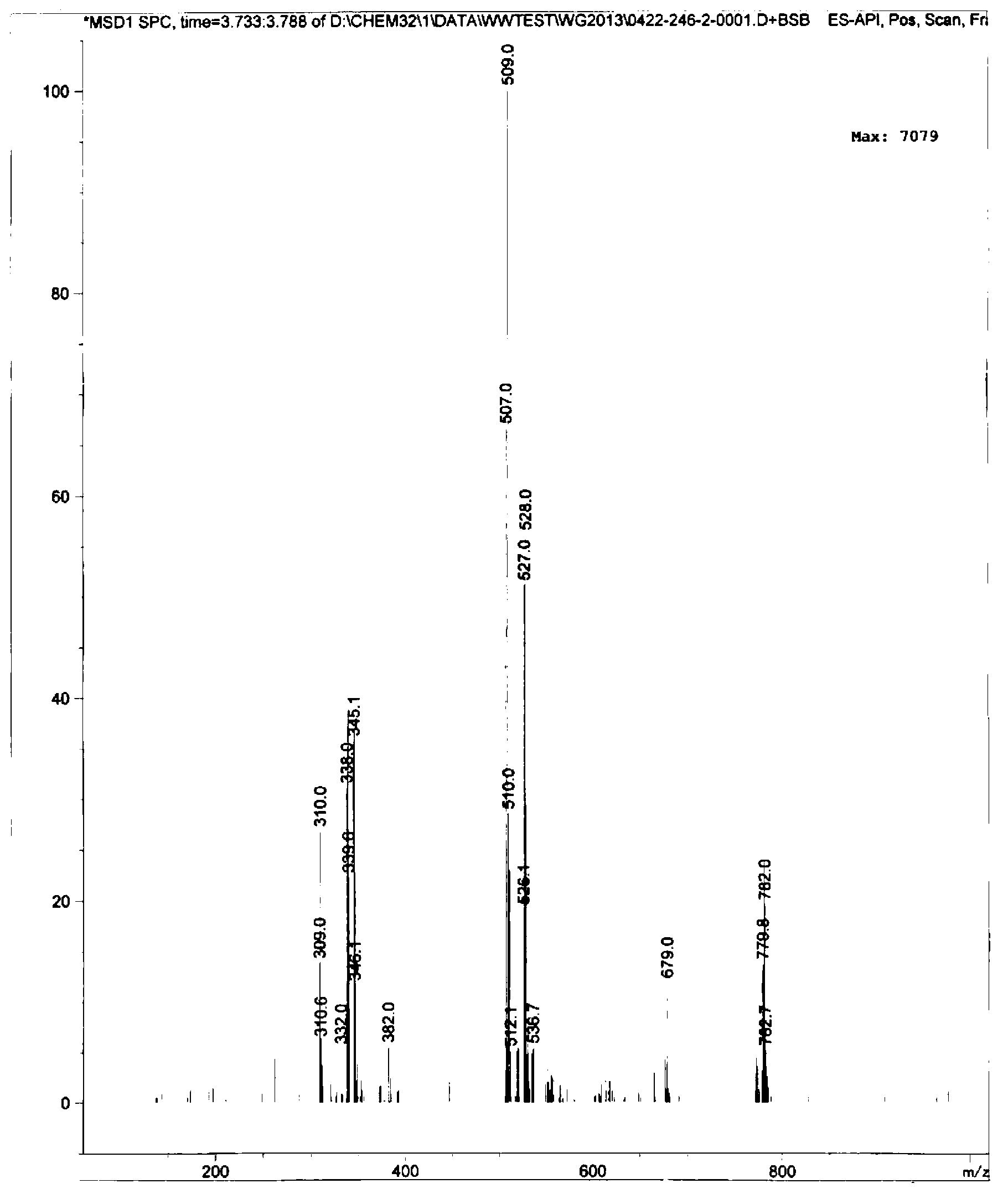
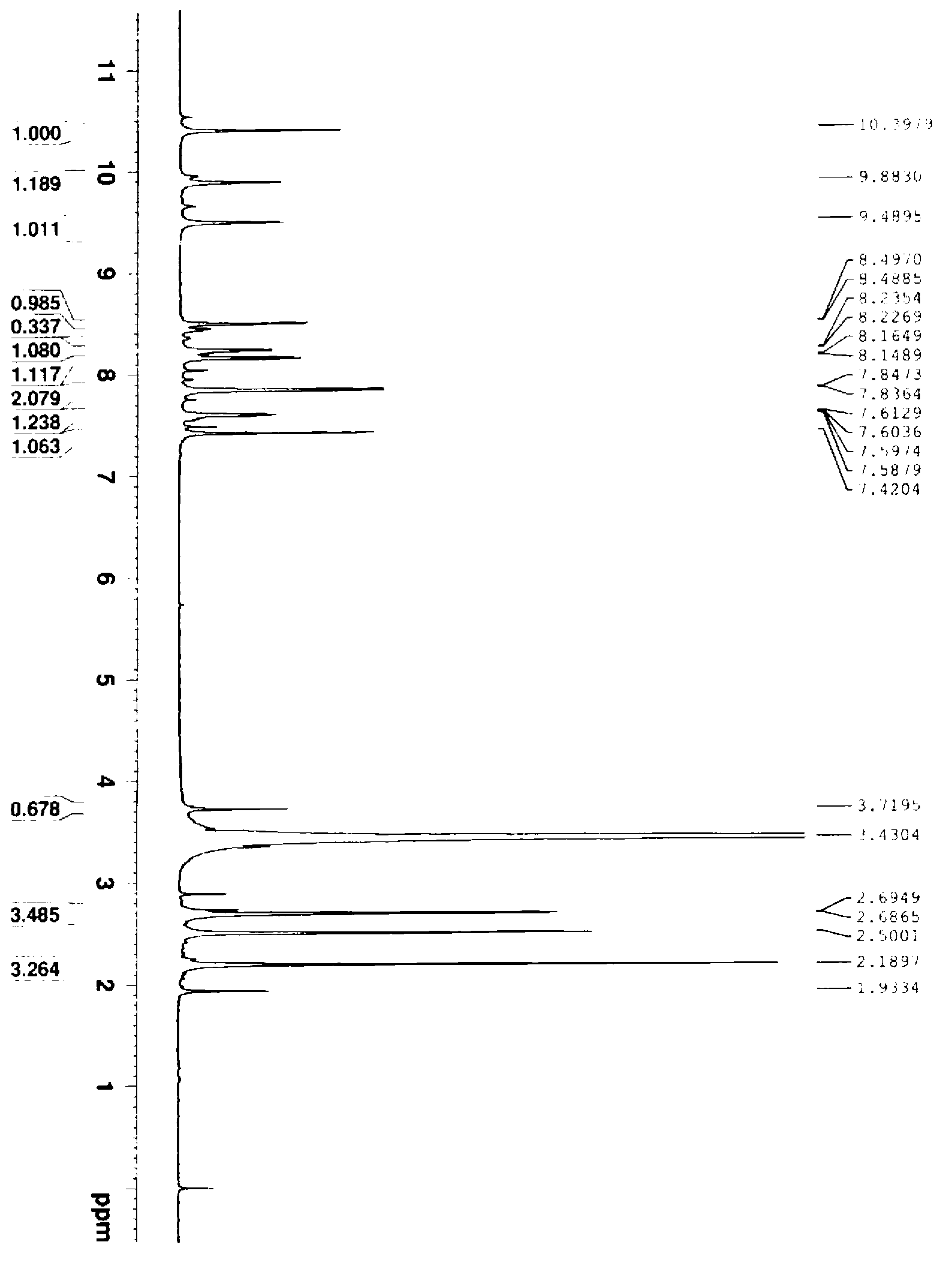
[0030] The chemical name of the anthranilamide compound in this embodiment is: 3-bromo-1-(3-chloro-2-pyridyl)-N-[4-thioformamide-2-methyl-6-( Methylcarbamoyl)-phenyl]-1H-pyrazole-5-carboxamide, its structural formula is as follows:
[0031]
[0032] The preparation method of this anthranilamide compound is as follows:
[0033] Add 3.0g of 70wt% NaSH (0.0375mol), 1.9g of magnesium chloride (0.02mol) and 50mL of ethylene glycol dimethyl ether into a 250mL four-neck flask, stir to dissolve completely, and then add 9.5g of bromcyanogen Add tebendamide (0.02mol) into the reaction system, raise the temperature to 25°C under stirring, and keep it warm for 10-11 hours.
[0034] After the reaction was completed, ethylene glycol dimethyl ether was distilled off under vacuum first, then 100 mL of water was added, and the pH was adjusted to 6-7 with hydrochloric acid, then stirred at below 10°C for 1 h, and filtered to obtain 9.8 g of a khaki-yellow crude product. The yield was 86.7%...
Embodiment 2~ Embodiment 4)
[0038] The compound of each example is the same as that of Example 1.
[0039] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 1.
[0040] Table 1
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com