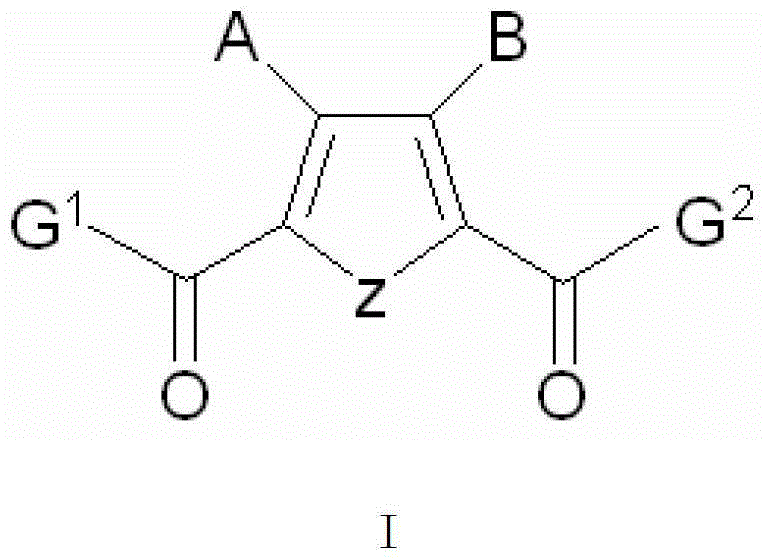
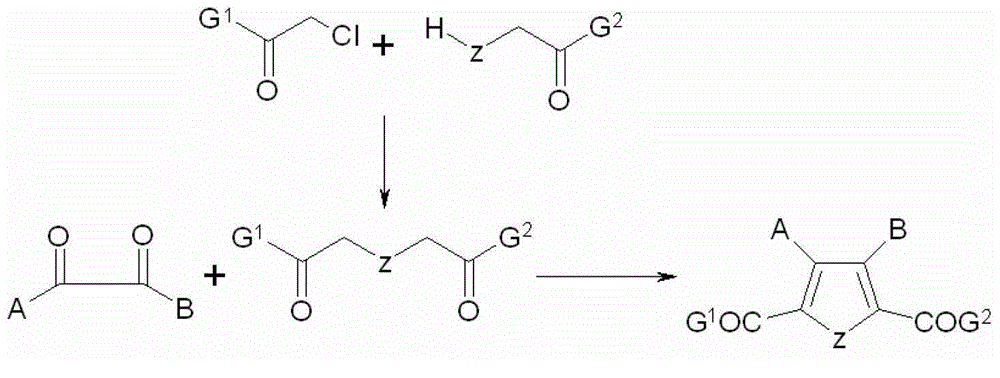
Five-membered heterocyclic dicarbonyl derivative and applications thereof for resisting multidrug-resistant bacteria
A kind of use and pharmaceutical technology, applied in the direction of antibacterial drugs, active ingredients of heterocyclic compounds, organic compounds, etc., to achieve the effect of anti-multidrug-resistant bacterial infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 4-Methyl-2-acetyl-5-propionyl-3-hydroxyfuran (CPU-ZH003)
[0046]Add 9ml (0.1mol) of propionyl chloride, 8.8ml (0.1mol) of 1-hydroxy-2-butanone and 14g of potassium carbonate (0.1mol) into a 100ml three-necked bottle, stir electromagnetically, and react under reflux at 80°C for 6 hours. Raise the reaction temperature to 110°C, slowly drop 8.5ml (0.1mol) of 2-oxopropionic acid into the reaction solution, and continue the reaction for 10 hours after the addition. Suction filtration, the reaction solution was concentrated under reduced pressure, and the remaining liquid was extracted with ethyl acetate. Concentrate and dry to obtain 10.56 g of solid, with a total yield of 53.86%.
[0047] Molecular formula: C 10 h 12 o 4 , molecular weight: 196.20
[0048] MS (EI) m / z 196.
[0049] 1 H-NMR (AV-500, δ, DMSO-d 6 ): 1.15(t,3H); 1.92(s,3H); 2.48(s,3H); 3.75(q,2H); 5.40(s,1H)
Embodiment 2
[0051] 3-((1-H-1,2,4-Triazolyl)methylene)-2-acetyl-3-hydroxy-pyrrolecarboxylic acid (CPU-ZH008)
[0052] Add 11.6g (0.1mol) of sodium chloroacetate, 7.5ml (0.1mol) of 1-amino-2-propanone and 14g of potassium carbonate (0.1mol) into a 100ml three-necked flask, stir it electromagnetically, and reflux at 95°C for 6 hours , raise the reaction temperature to 110°C, slowly drop 15g (0.1mol) of 1-H-1,2,4-triazolyl-2-oxopropionic acid into the reaction liquid, react overnight, add concentrated hydrochloric acid to The pH of the solution was about 4.0. Suction filtration, the reaction solution was concentrated under reduced pressure, and the remaining liquid was extracted with a mixed organic solvent of ethyl acetate: chloroform = 3:1. Concentrate and dry to obtain 18.14 g of solid, with a total yield of 71.13%.
[0053] Molecular formula: C 10 h 10 N 4 o 4 , molecular weight: 250.22
[0054] MS (EI) m / z 249 (M-1).
[0055] 1 H-NMR (AV-500, δ, DMSO-d 6 ):2.08(t,3H);5.02(s,1H)...
Embodiment 3
[0057] Dimethyl 3,4-dibenzyloxy-2,5-thiophenedicarboxylate (CPU-ZH016)
[0058] Add 8ml (0.1mol) of acetyl chloride, 0.1mol of 1-mercapto-2-acetone, 200ml of DMF and 14g of potassium carbonate (0.1mol) into a 250ml three-necked bottle, stir with electromagnetic force, react at 40°C for 3 hours, and raise the reaction temperature At 85°C, 27 g (0.1 mol) of dibenzyl oxalate was slowly added to the reaction liquid, and the reaction was carried out for 5 hours. Suction filtration, the reaction solution was concentrated under reduced pressure, and the remaining liquid was extracted with chloroform. Concentrate and dry to obtain 20.14 g of solid, with a total yield of 53.02%.
[0059] Molecular formula: C 22 h 20 o 6 S, molecular weight: 412.47
[0060] MS (EI) m / z 435.1 (M+23)
[0061] 1 H-NMR (AV-500, δ, DMSO-d 6 ): 3.00(s,3H); 5.16(s,2H); 7.24-7.41(m,5H); 7.83(s,2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com