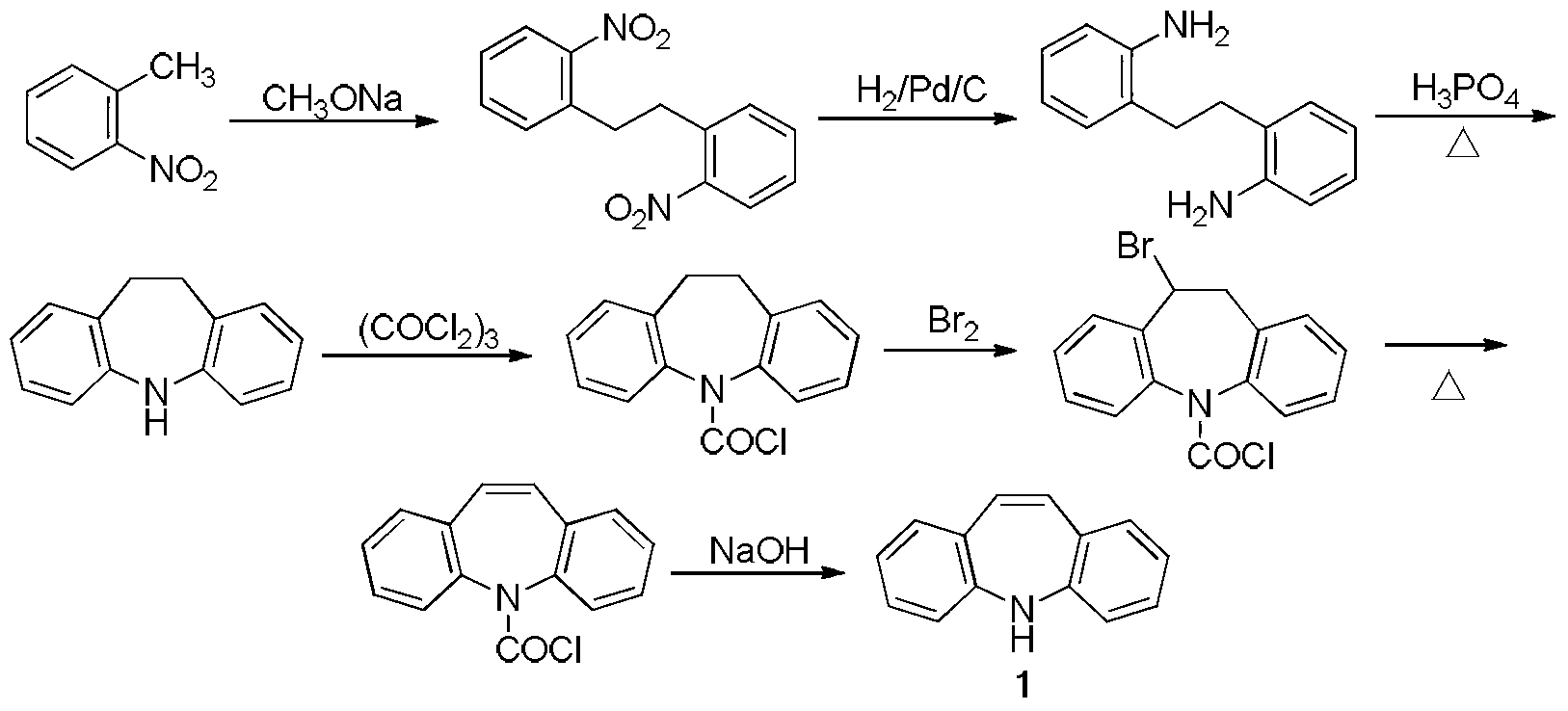
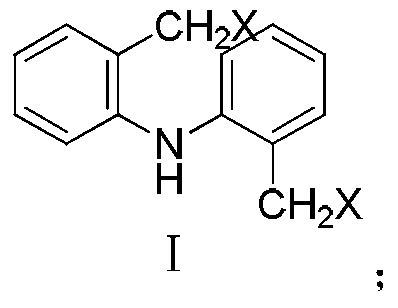
Synthesis method of iminostilbene
An iminostilbene and synthesis method technology, applied in the direction of organic chemistry and the like, can solve the problems of heavy pollution, long steps, low yield and the like, and achieve the effects of optimizing reaction conditions and shortening reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Add 6.65 g (0.025 mol) of 2-(dichlorotoluene) diphenylamine, 0.14 g (0.0012 mol) of L-proline and 10 mL of absolute ethanol to a 100 mL four-necked flask in sequence. Stir and keep the temperature at 20-40°C, add potassium hydroxide absolute ethanol solution (containing 2.80g, 0.05molKOH, 27.50mL CH 3 CH 2 OH). The reaction time is 5-12 hours. Filter, wash the brown solid with absolute ethanol three times, extract with hot ethyl acetate, cool, the solid precipitates, and recrystallize with toluene to obtain 2.19 g of a golden yellow solid with a yield of 45.38%.
example 2
[0023] Add 8.87 g (0.025 mol) of 2-(dibromotoluene) diphenylamine, 0.58 g (0.0050 mol) of L-proline and 20 mL of absolute ethanol to a 100 mL four-neck flask in sequence. Stir and keep the temperature at 20-40°C, add potassium hydroxide absolute ethanol solution (containing 7.01g, 0.12molKOH, 42.50mL CH 3 CH 2 OH). The reaction time is 5-12 hours. Filter, wash the brown solid with absolute ethanol three times, extract with hot ethyl acetate, cool, the solid precipitates, and recrystallize with toluene to obtain 2.92 g of a golden yellow solid with a yield of 60.51%.
example 3
[0025] 11.22 g (0.025 mol) of 2-(diiodotoluene) diphenylamine, 0.44 g (0.0038 mol) of L-proline and 20 mL of absolute ethanol were sequentially added into a 100 mL four-necked flask. Stir and keep the temperature at 20-40°C, add potassium hydroxide absolute ethanol solution (containing 4.90g, 0.087molKOH, 30mL CH 3 CH 2 OH). The reaction time is 5-12 hours. Filter, wash the brown solid with absolute ethanol three times, extract with hot ethyl acetate, cool, the solid precipitates, and recrystallize with toluene to obtain 3.90 g of a golden yellow solid with a yield of 80.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com