Amphiphilic acid-sensitive ternary molecular brush polymer constructed acid-sensitive nanocapsule
A ternary molecular brush and nanocapsule technology is applied in the field of self-assembled polymer materials to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] An amphiphilic acid-sensitive ternary molecular brush polymer is prepared by the following steps:
[0045] (1) P(GMA-N 3 ) main chain synthesis
[0046] Take 2 parts of ethyl 2-bromoisobutyrate initiator, 200 parts of glycidyl methacrylate (GMA), 100 parts of diphenyl ether, 2 parts of CuBr and 2 parts of N,N,N',N',N" -Pentamethyldiethylenetriamine (PMDETA), conduct ATRP reaction at 30°C for 1.5 hours under nitrogen protection to obtain polyglycidyl methacrylate (PGMA) with a degree of polymerization (DP) of 60.
[0047] Take 100 parts of PGMA (DP=60), 100 parts of NaN 3 , 500 parts of dimethylformamide (DMF) and 1 part of AlCl 3 , reacted at 50°C for 24 hours to obtain P(GMA-N 3 ), as the main chain.
[0048] (2) Synthesis of three side chains
[0049] Synthesis of hydrophilic side chains: Take 100 parts of monomethoxypolyethylene glycol (Mn=5000), 20 parts of 2-propynylacetic acid, 20 parts of 4-dimethylaminopyridine (DMAP), 20 parts of 1-( 3-Dimethylaminopropy...
Embodiment 2
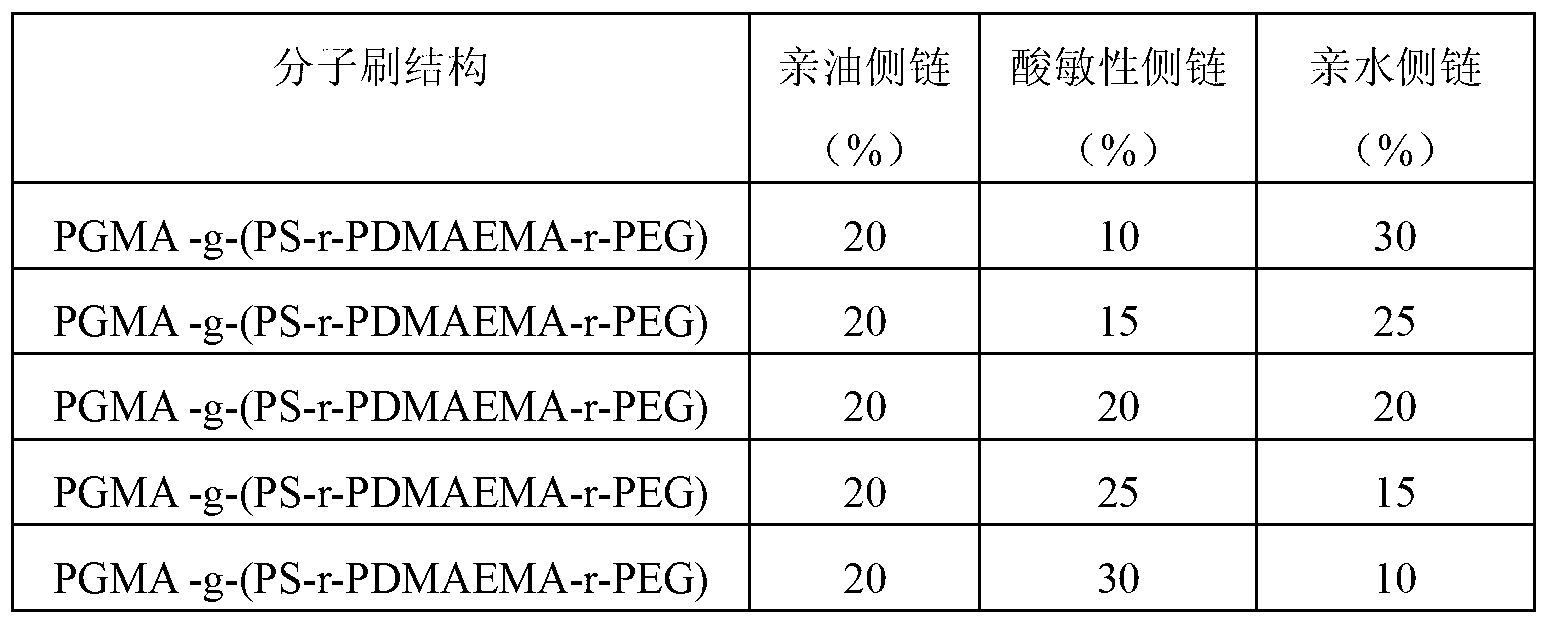
[0058] The preparation method and raw material composition are the same as in Example 1, only the grafting rate of the hydrophilic side chain and the acid-sensitive side chain of the amphiphilic acid-sensitive ternary molecular brush polymer of Example 1 is adjusted to obtain Acid-sensitive nanocapsules with different structures, so as to obtain acid-sensitive nanocapsules that do not drive the response performance. The graft ratios of the three side chains are shown in Table 1.
[0059] Table 1: Synthesis of amphiphilic acid-sensitive ternary molecular brush polymers with different structures
[0060]
[0061] The acid-sensitive nanocapsules were constructed from the amphiphilic acid-sensitive ternary molecular brush polymers synthesized in Table 1, and the acid-sensitive properties of the constructed acid-sensitive nanocapsules became stronger with the increase of the grafting amount of PDMAEMA.
Embodiment 3
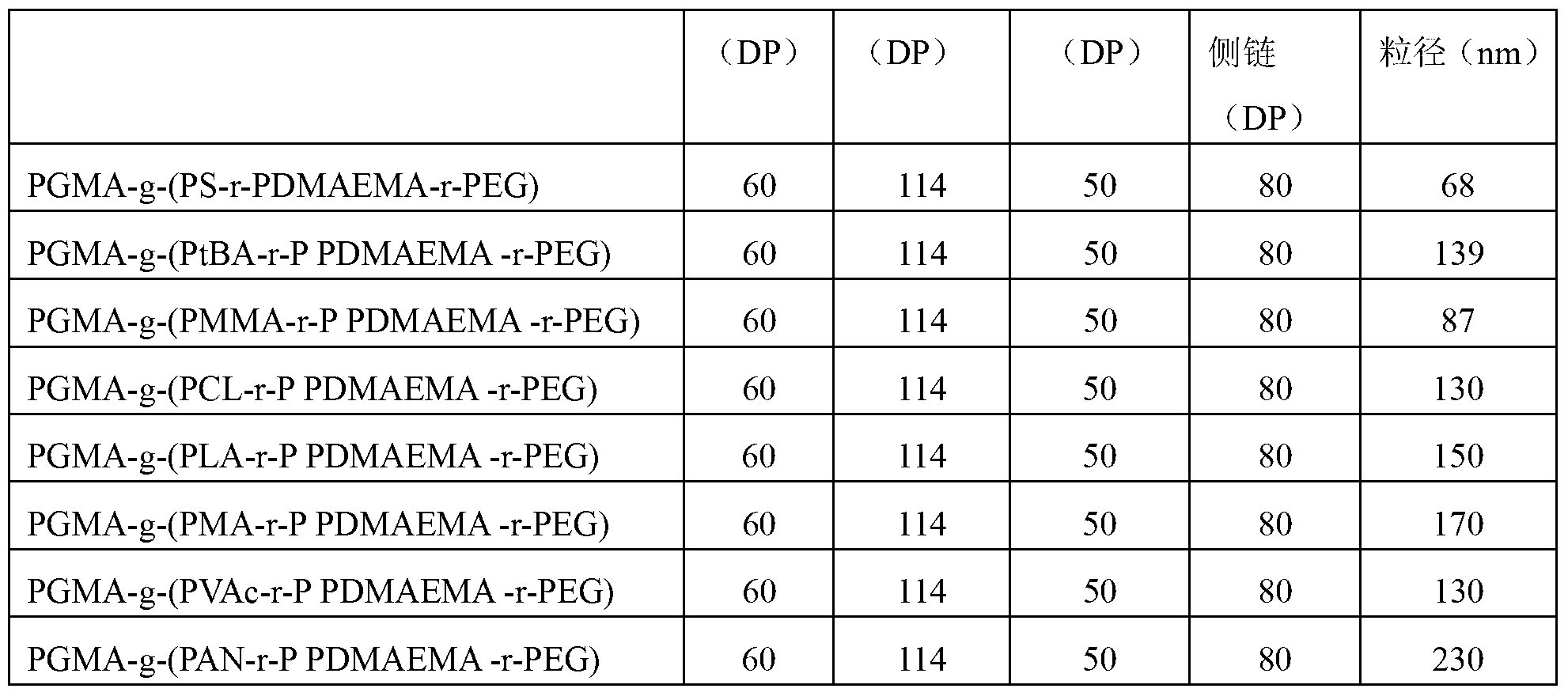
[0063] The preparation method and raw material composition are the same as in Example 1, only changing the composition of the lipophilic side chain of the amphiphilic acid-sensitive ternary molecular brush polymer in Example 1, acid-sensitive nanocapsules with different particle sizes can be prepared . The composition of the lipophilic side chain and the particle size of the nanocapsules are shown in Table 2.
[0064] The preparation method of PtBA, PMMA, PCL, PLA, PMA, PVAc, and PAN with a degree of polymerization of lipophilic side chain of 50 is similar to that of PS, and is prepared by commonly used ARTP.
[0065] The grafting ratios of PEG, PDMAEMA, and lipophilic side chains of each amphiphilic polymer molecular brush were 15%, 25%, and 20%, respectively.
[0066] Table 2: Effect of lipophilic side chain composition of molecular brushes on particle size of acid-sensitive nanocapsules
[0067]
[0068]
[0069] It can be seen from Table 2 that acid-sensitive nanoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com