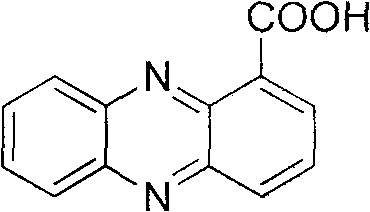
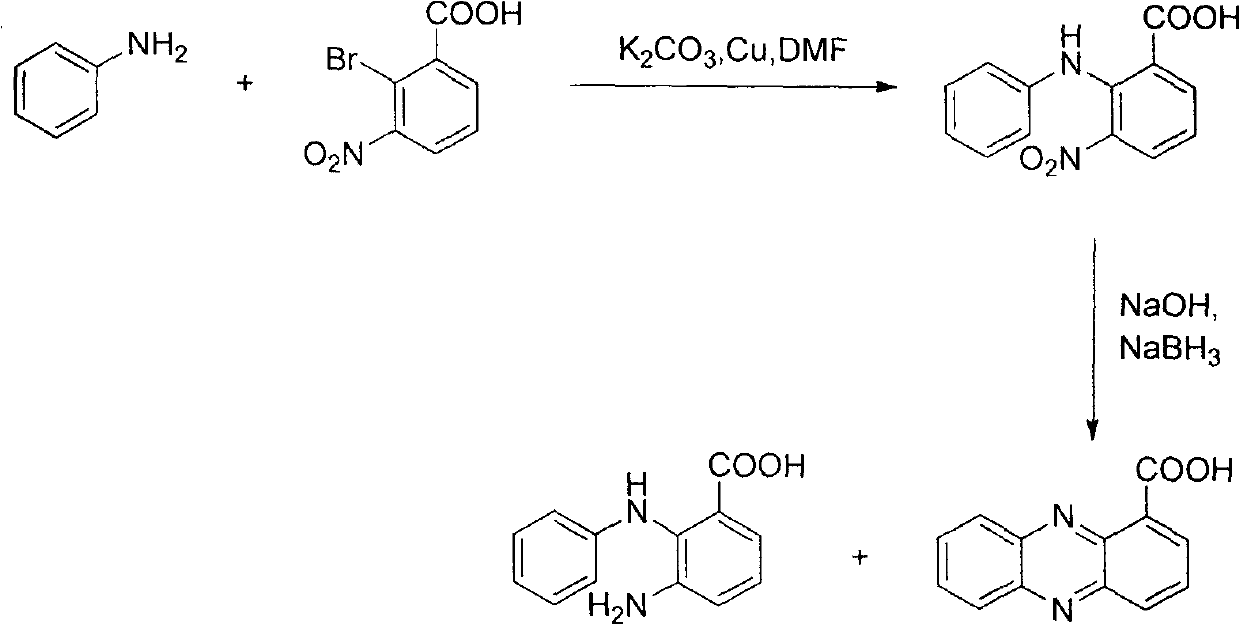
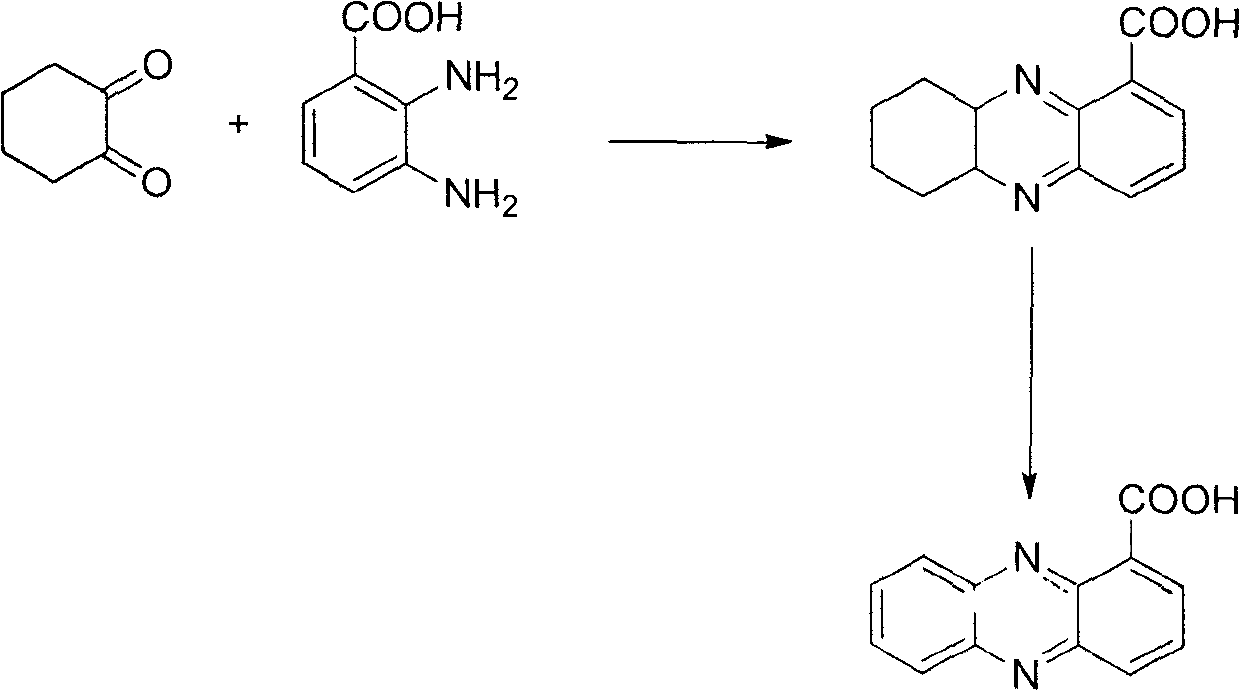
Method for synthesizing phenazine-1-carboxylic acid
A certain amount of shenzimycin, which is applied in the field of synthesis of the microbial-derived pesticide shenzimycin, can solve the problems of harsh conditions, unsuitability for industrial production, and low yield in the second step.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of 1-methyl-5,10-dihydrophenazine
[0032] In the glove box of nitrogen atmosphere, weigh catechol (1.1g, 10mmol), 2,3-diaminotoluene (1.22g, 10mmol), then pour into mortar, grind and mix well, transfer the mixture to In a 50ml four-neck flask, under magnetic stirring, heat up to 210°C, melt, and keep warm for 35h. Cool to below 100°C, add hot water, stir for about 2 hours, and filter with suction. The filter cake was washed several times with 80°C water and dried to obtain a yellow-green solid. Petroleum ether was recrystallized to obtain 1.26 g of pale yellow-green crystals. Yield 64.2%, melting point 181-182°C.
Embodiment 2
[0033] Embodiment 2: the preparation of 1-methyl-5,10-dihydrophenazine
[0034] In the glove box of nitrogen atmosphere, take catechol (1.32g, 12mmol), 2,3-diaminotoluene (1.22g, 10mmol), then pour into mortar, fully grind and mix, and transfer the mixture to In a 50ml four-neck flask, under magnetic stirring, heat up to 190°C, melt, and keep warm for 40h. Cool to below 100°C, add hot water, stir for about 2 hours, and filter with suction. The filter cake was washed several times with 80°C water and dried to obtain a yellow-green solid. Petroleum ether was recrystallized to obtain 1.43 g of light yellow-green crystals. Yield 73.1%, melting point 181-182°C.
Embodiment 3
[0035] Embodiment 3: the preparation of 1-methyl-5,10-dihydrophenazine
[0036] In a glove box under a nitrogen atmosphere, weigh catechol (1.1g, 10mmol), 2,3-diaminotoluene (1.46g, 12mmol), pour into a mortar, grind and mix well, and transfer the mixture to In a 50ml four-neck flask, under magnetic stirring, heat up to 150°C, melt, and keep warm for 50h. Cool to below 100°C, add hot water, stir for about 2 hours, and filter with suction. The filter cake was washed several times with 80°C water and dried to obtain a yellow-green solid. Petroleum ether was recrystallized to obtain 1.31 g of pale yellow-green crystals, with a yield of 66.9% and a melting point of 181-182°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com