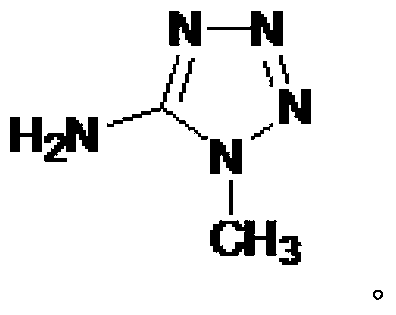
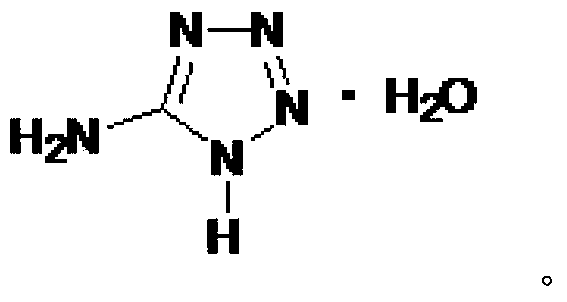
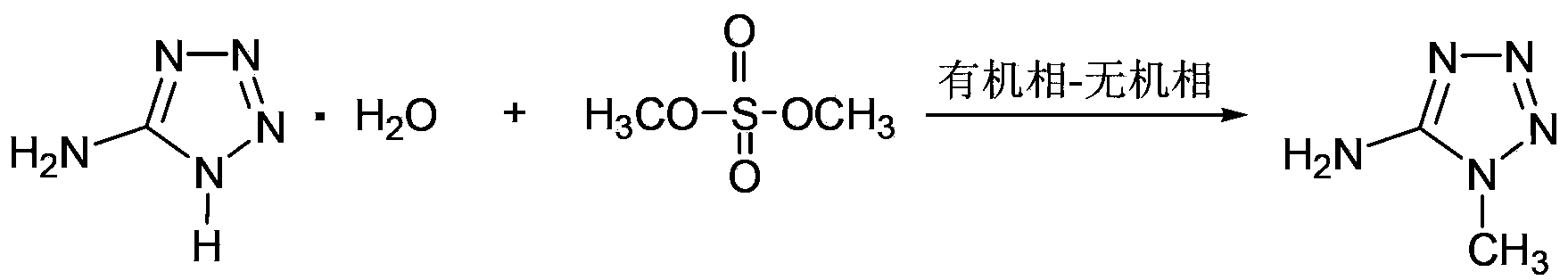Synthesizing method of 1-methyl-5-aminotetrazole
A technology of aminotetrazole and synthesis method, applied in directions such as organic chemistry, can solve the problems of many by-products, low reaction yield and the like, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 20ml of distilled water and 15.4g of 5-aminotetrazole monohydrate into a 250ml three-neck flask to form a suspension. Under stirring at 20°C to 25°C, add 89ml of aqueous sodium hydroxide solution with a mass concentration of 7.0% into the three-necked flask, and stir to dissolve 5-aminotetrazole monohydrate. Mix 9.8g of dimethyl sulfate with 20ml of toluene, add the toluene liquid containing dimethyl sulfate into the three-necked flask under stirring at 20°C~25°C, heat up to 88°C~89°C while stirring, and react for 1.5 hours , cooled to room temperature, the reaction solution was left to stand, and the water phase was separated. After about 70ml of water was evaporated under reduced pressure, the water phase after part of the water was evaporated under reduced pressure was cooled to 0 ° C ~ 3 ° C, filtered, and the obtained solid was filtered with 0 The solid was washed with 5 ml of cold water at ℃~3℃, and dried at room temperature to obtain a crude product. Accordi...
Embodiment 2
[0035] Add 20ml of distilled water and 15.4g of 5-aminotetrazole monohydrate into a 250ml three-neck flask to form a suspension. Under stirring at 20°C to 25°C, add 83ml of aqueous sodium hydroxide solution with a mass concentration of 7.5% into the three-necked flask, and stir to dissolve 5-aminotetrazole monohydrate. Mix 9.8g of dimethyl sulfate with 60ml of xylene, add the xylene liquid containing dimethyl sulfate into the three-necked flask under stirring at 20°C to 25°C, heat up to 92°C to 93°C while stirring, and react After 2.5 hours, cool to room temperature, let the reaction solution stand still, separate the water phase, evaporate about 70ml of water under reduced pressure, cool the water phase after removing part of the water under reduced pressure to 0°C-3°C, filter, and filter the obtained solid, The solid was washed with 5 ml of cold water at 0°C to 3°C, and dried at room temperature to obtain a crude product. According to NMR analysis, 1-methyl-5-aminotetrazole...
Embodiment 3
[0037]Add 20ml of distilled water and 15.4g of 5-aminotetrazole monohydrate into a 250ml three-neck flask to form a suspension. Under stirring at 20°C to 25°C, add 87ml of aqueous sodium hydroxide solution with a mass concentration of 7.2% into the three-necked flask, and stir to dissolve 5-aminotetrazole monohydrate. Mix 9.8g dimethyl sulfate with 100ml mixed solvent (toluene: chlorobenzene=1:1, v / v), mix the mixed solvent of toluene and chlorobenzene containing dimethyl sulfate under stirring at 20℃~25℃ Add the liquid into the three-necked flask, heat it up to 91°C to 93°C under stirring, react for 4.5 hours, cool to room temperature, let the reaction liquid stand still, separate the water phase, and evaporate about 70ml of water under reduced pressure, and then remove part of the water under reduced pressure The final aqueous phase was cooled to 0°C-3°C, filtered, and the obtained solid was filtered, washed with 5ml of cold water at 0°C-3°C, and dried at room temperature to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com