Process for enhanced acid leaching of laterite ores
A technology for leaching and ore, which is applied in the field of leaching nickel-containing laterite ore, can solve the problems of negative environmental impact, slowness, high acid consumption, etc., and achieve the effect of reducing demand, providing economic benefits, reducing or eliminating the release of acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
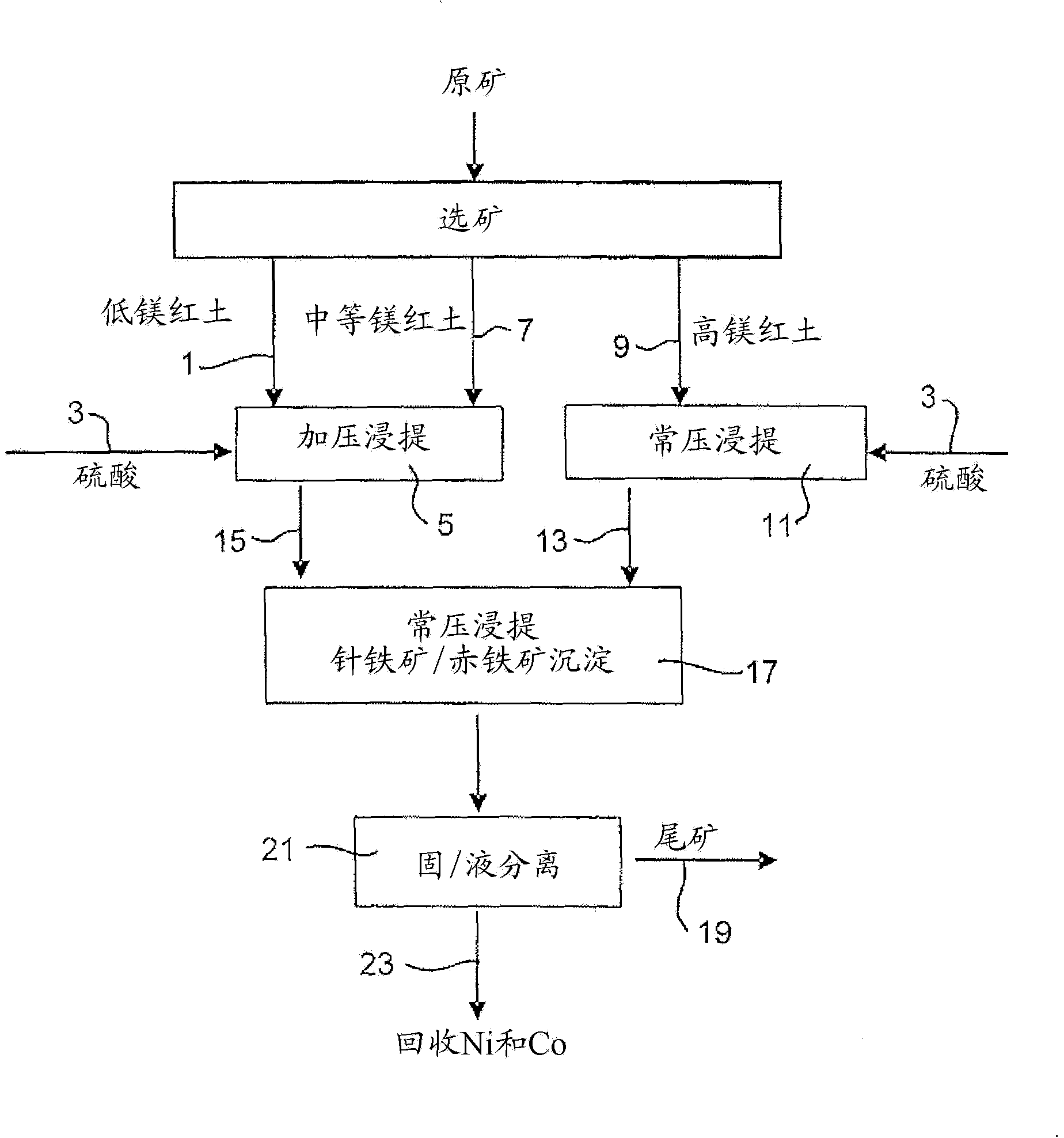
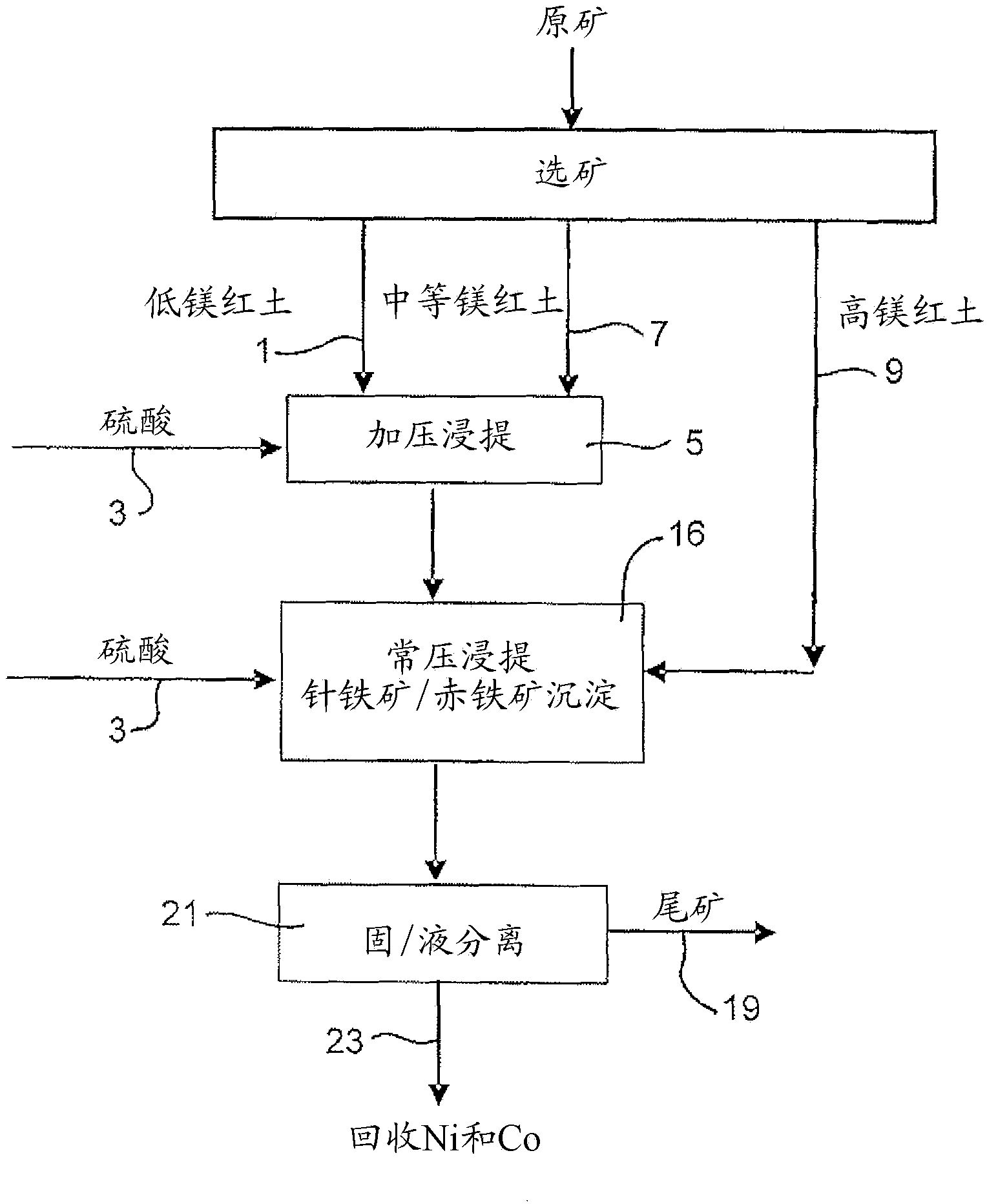
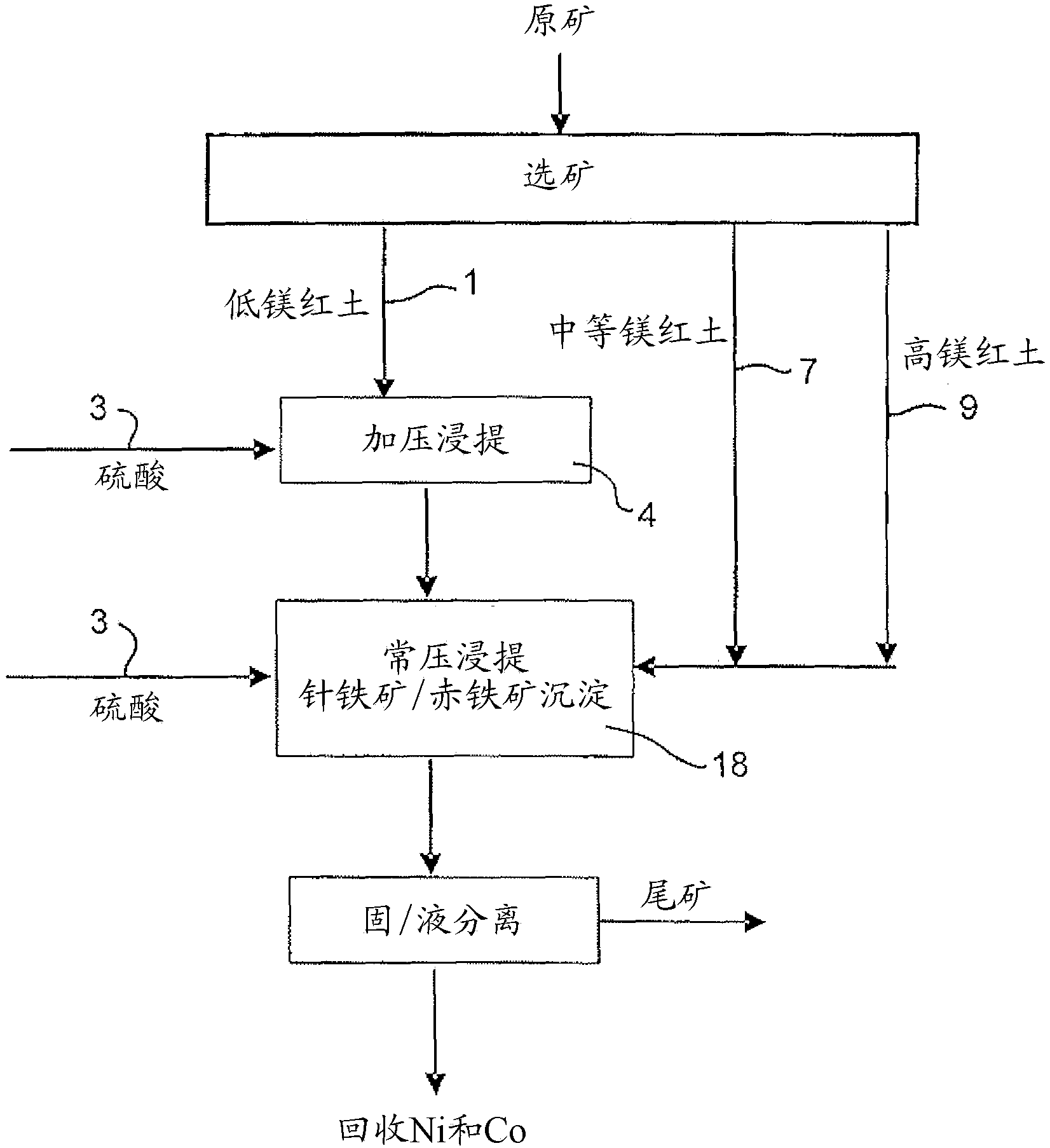
Image
Examples
Embodiment 1
[0056] Example 1: Ore Treatment, Chemical Analysis and Mineralogical Research
[0057] The three limonite samples were stirred in tap water for 2 hours and passed through a 1 mm sieve. Put any too large substance in Na, K and NH 4 Water with low ion content is ground to less than 1 mm with a rod mill. The two saprolite samples were subjected to Na, K and NH 4 Grinding with a rod mill in water with low ion content to P 80 100 <650μm. The slurries of limonite and saprolite were adjusted to solids concentrations of 30% w / w and 25% w / w, respectively. The SG and true PSD (particle size distribution) of the ore were measured with a Malvern Instrument, see Table 1.
[0058] Table 1: SG and PSD of Feed Ore
[0059]
[0060] The chemical analysis results of the ore samples are listed in Table 2.
[0061] Table 2: Chemical analysis of laterite samples
[0062]
[0063] The results of the mineralogical studies of the ore samples are briefly summarized in Table 3.
[0064] ...
Embodiment 2
[0066] Example 2: Continuous pressure leaching of limonite 1 containing 4.9% magnesium and normal pressure leaching of saprolite 2
[0067] 914 g of a 30.3% w / w limonite 1 slurry (as in Example 1) and 118 g of 98% H 2 SO 4 Added to a 2 liter titanium autoclave. In a stirred autoclave, pressurized leaching was continued for 1 hour at 250 °C and 48 bar (heating time removed). Simultaneously, 1101 g of 25.2% w / w saprolite 2 slurry (as shown in Example 1) and 159 g of 98% H 2 SO4 Mixed in a 3 liter stirred glass reactor and leached for 30 minutes at 95°-104°C and atmospheric pressure. The saprolite was heated to 60°C before adding the acid. The acidities of the final solutions obtained by pressure leaching of limonite and atmospheric pressure leaching of saprolite were 38.3g / L and 15.7g / L, respectively. The pressure leach slurry was transferred to the glass reactor while hot (~90°C) and mixed with the saprolite leach slurry, and atmospheric leaching and precipitation of iron ...
Embodiment 3
[0072] Example 3: Continuous pressure leaching of limonite 2 containing 2.7% magnesium and normal pressure leaching of saprolite 2
[0073] 914 g of 30.5% w / w Limonite 2 slurry (as shown in Example 1) and 104 g of 98% H 2 SO 4 Mixed in a 2 liter stirred titanium autoclave. In the autoclave, pressure leaching was continued for 1 hour at 250°C and 48 bar (heating time excluded). Simultaneously, 1101 g of 25.2% w / w saprolite 2 slurry (as shown in Example 1) and 181 g of 98% H 2 SO 4 Mixed in a 3 liter stirred glass reactor and leached for 30 minutes at 95°-104°C and atmospheric pressure. The saprolite was heated to 60°C before adding the acid. The acidities of the final solutions obtained by pressure leaching of limonite and atmospheric pressure leaching of saprolite were 46.1g / L and 22.6g / L, respectively. The pressure leaching slurry was transferred to the glass reactor while it was hot (~90°C) and mixed with the saprolite leaching slurry, and the atmospheric leaching and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com