Mosapride citrate dehydrate sustained release tablet
A technology for mosapride citrate and sustained-release tablets, which is applied to medical preparations of non-active ingredients, digestive system, organic active ingredients, etc. It can solve the problems that sustained-release preparations are not necessarily suitable and the preparation process is complicated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Prescription:
[0017] Mosapride citrate
15g
HPMC K4M
20g
HPMC K15M
15g
60g
80% ethanol solution
Appropriate amount
2g
Opadry
2g
A total of 1000 pieces were made
[0018] 2. Process steps:
[0019] Take mosapride citrate, pulverize it and pass through a 100-mesh sieve, stir it with lactose in a high-speed mixer for 5 minutes, moisten it with 80% ethanol and granulate it, dry it at 50°C, and control the moisture content of the granules to less than 0.5 %, the granules pass through a 40-mesh sieve for granulation, add HPMC K4M, HPMC K15M, and magnesium stearate to mix evenly and then press into tablets. The prepared plain tablets are coated with Opadry, the tablet bed temperature is controlled at 40-45°C, the atomization pressure is 0.2-0.3MPa, and the weight gain of the coated tablets is 2-2.5%.
[0020] Release measurement method:...
Embodiment 2
[0030] 1. Prescription:
[0031] Mosapride citrate
15g
HPMC K4M
20g
HPMC K15M
10g
lactose
50g
80% ethanol solution
Appropriate amount
Magnesium stearate
2g
Opadry
2g
A total of 1000 pieces were made
[0032] 2. Process steps:
[0033] With embodiment 1;
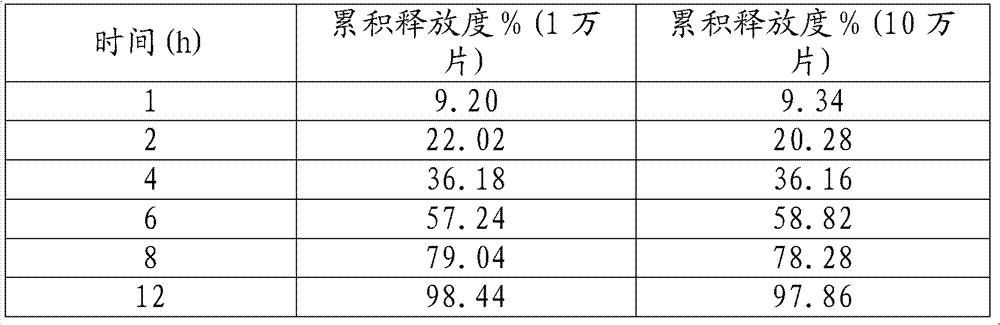
[0034] The release measurement results are as follows:
[0035] time (h)
cumulative release %
1
9.14
2
20.14
4
35.16
6
56.88
8
78.20
12
97.24
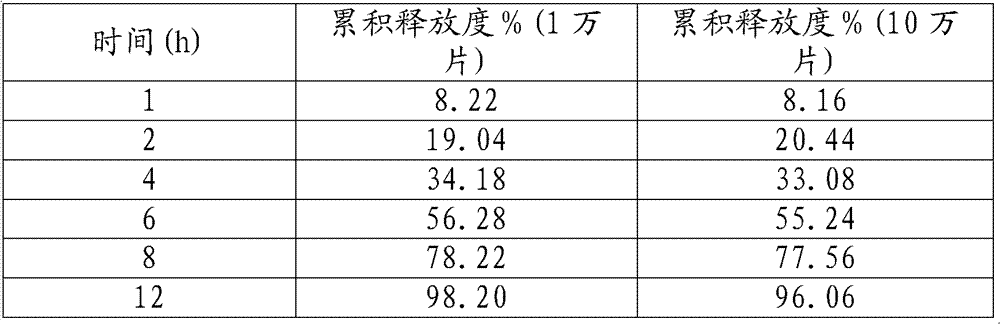
[0036] In production, according to the prescription and process, the release of 10,000 and 100,000 samples of samples is as follows:
[0037]
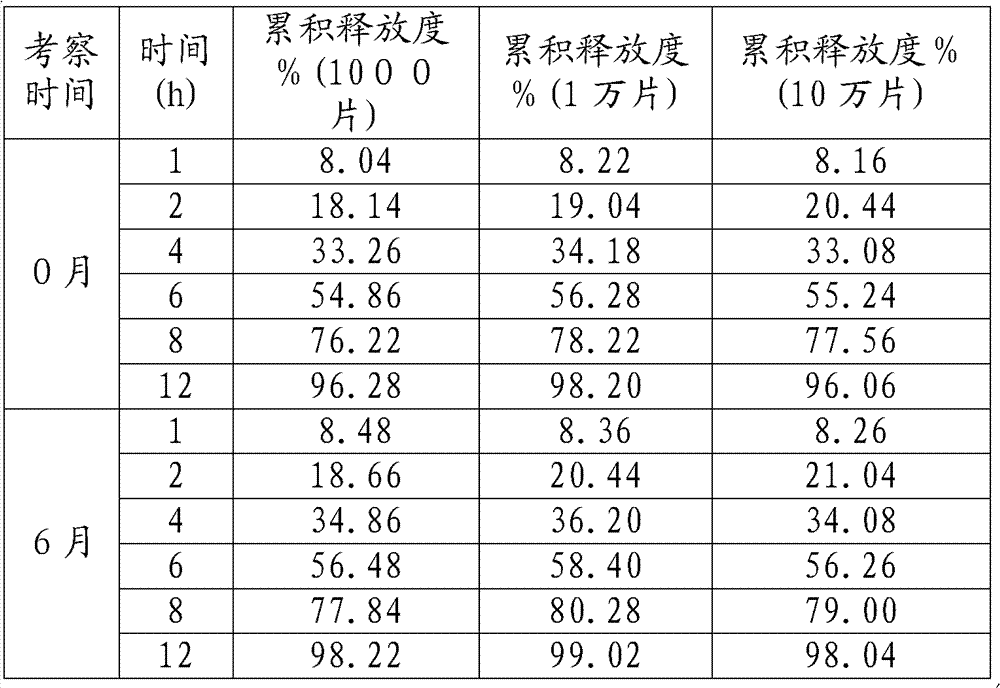
[0038] 1000 pieces, 10,000 pieces, and 100,000 pieces of samples were respectively inspected through accelerated tests. Acceleration conditions: temperature: 40 degrees, humidity: RH75%;
[0039] The release results are ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com