Mosapride Citrate Sustained Release Tablets
A technology for mosapride citrate and sustained-release tablets, which is applied in the direction of digestive system, pharmaceutical formulations, drug combinations, etc., and can solve the problems that sustained-release preparations are not necessarily suitable and the preparation process is complicated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Prescription:
[0017] Mosapride Citrate
15g
HPMC K4M
20g
HPMC K15M
15g
60g
80% ethanol solution
Moderate
2g
Opadry
2g
A total of 1000 pieces were made
[0018] 2. Process steps:
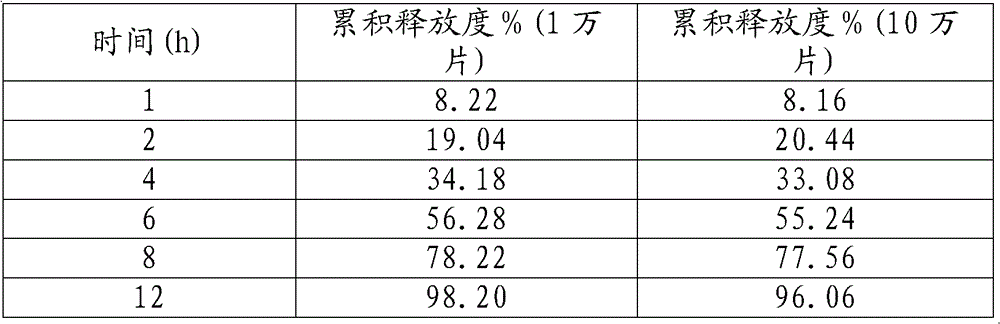
[0019] Take mosapride citrate, pulverize and pass through a 100-mesh sieve, stir with lactose at a high speed in a high-speed mixer for 5 minutes, wet it with 80% ethanol, granulate, and dry at 50 °C. After drying, the moisture of the granules is controlled to be less than 0.5 %, the granules pass through a 40-mesh sieve and granulate, add HPMCK4M, HPMCK15M, and magnesium stearate to mix evenly, and then press into tablets. The prepared plain tablet is coated with Opadry, the temperature of the tablet bed is controlled at 40-45 DEG C, the atomization pressure is 0.2-0.3 MPa, and the weight gain of the coated tablet is 2-2.5%.
[0020] Determination method of releas...
Embodiment 2
[0030] 1. Prescription:
[0031] Mosapride Citrate
15g
HPMC K4M
20g
HPMC K15M
10g
50g
80% ethanol solution
Moderate
Magnesium stearate
2g
Opadry
2g
A total of 1000 pieces were made
[0032] 2. Process steps:
[0033] With embodiment 1;
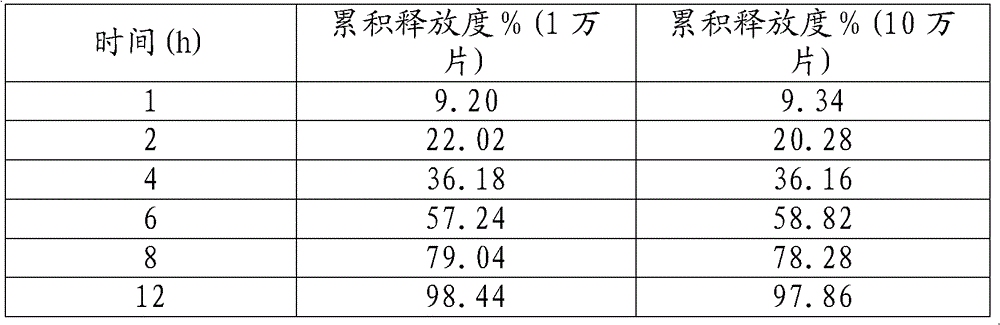
[0034] The release measurement results are as follows:
[0035] time (h)
Cumulative release %
1
9.14
2
20.14
4
35.16
6
56.88
8
78.20
12
97.24
[0036] In production, the release degrees of 10,000 and 100,000 samples prepared according to the prescription and process are as follows:
[0037]
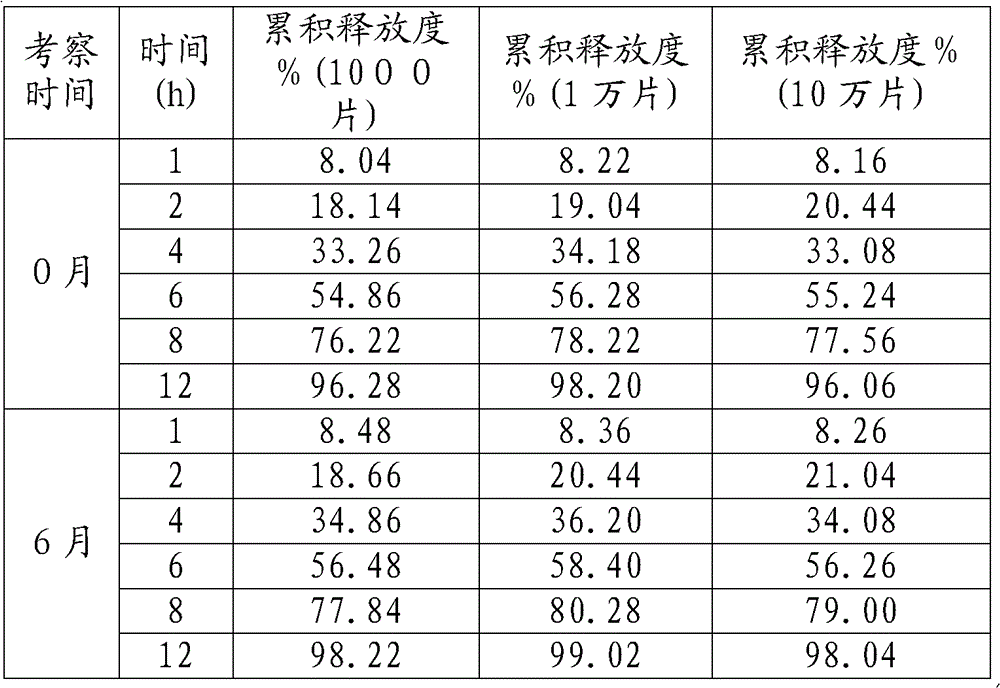
[0038] The samples of 1000 pieces, 10000 pieces and 100000 pieces were inspected by accelerated test respectively. Accelerated conditions: temperature: 40 degrees, humidity: RH75%;
[0039] The release results are as follows:
[0040]
[0041] Chemical sta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com